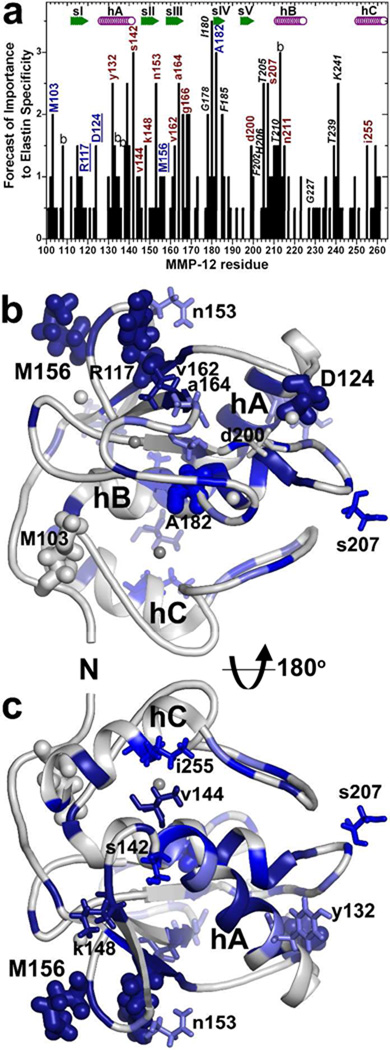
Fig. 1.
MMP-12 residues distal from the active site chosen for mutagenesis based on putative contacts with 20 kD α-elastin species (Eln-20) and BINDSIght forecast of importance (16): (a) Residues were selected for mutagenesis based on this BINDSIght plot suggesting interfaces that could be distinctive. Those with blue and red-brown labels were characterized in this study of remote surfaces and those in black italics in the prior study of the active site periphery (16). The score in the plot accumulated with NMR evidences of surfaces that are covered or perturbed in chemical shift by the Eln-20 binding partner and distinctions in sequence (16). Uppercase labels mark residues found to support higher catalytic efficiency toward fEln-100 than a conservative mutation, and lowercase for those that do not. Those underlined are consequential positions distant from the active site. “b” denotes a buried residue. In the front view (b) and back view (c) of the catalytic domain, dark blue, medium blue, and light blue represent, respectively, strong, medium, and mild protection of the surface by Eln-20 from an exogenous Gd·EDTA probe. Side chains are plotted for residues mutated and characterized in this study. Side chains that affected digestion of 100 kD α-elastin (fEln-100) are drawn with thick sticks, and those that did not with thin sticks. Zinc ions are darker gray and calcium lighter gray.