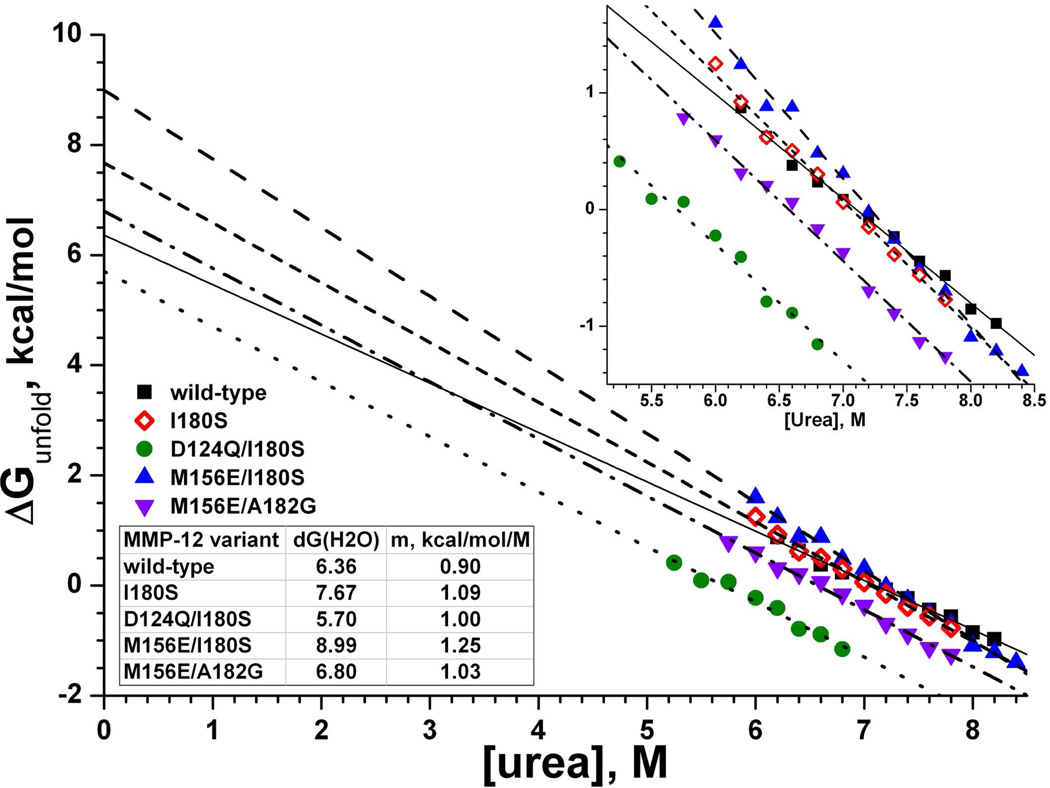
Fig. 5.
Folding stabilities of selected enzyme variants under chemical denaturation at 37 ºC. ΔG(H2O) is the free energy to unfold the protein in absence of denaturant accessible by linear extrapolation from the linear unfolding transition region to the Y-intercept. The fitted slope of [urea] dependence is m in eq. 4 (49). The reproducibility of extrapolated ΔG(H2O) observed was 1σ ≈ 3%. The inset shows an expanded view of the transition region.