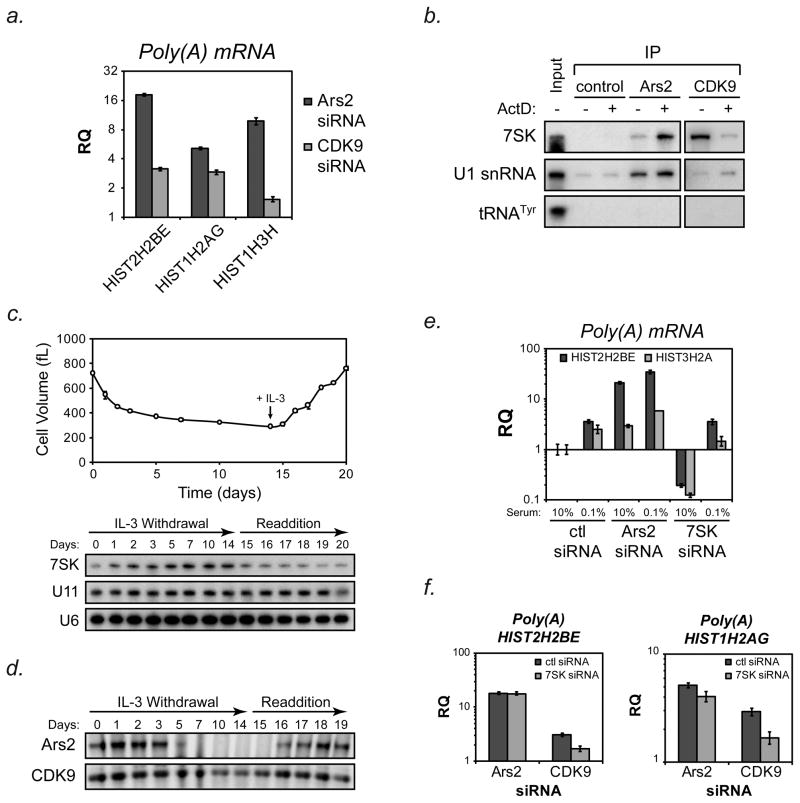
Figure 5. Reciprocal interactions of 7SK RNA with Ars2 and CDK9.
(A) Hela cells were transfected with siRNA targeting Ars2 (Ars2-2), CDK9 or a control siRNA and RNA was isolated three days later. cDNA synthesis was performed using oligo(dT) primers and TaqMan®-based qPCR was used to determine changes in the levels of polyadenylated HIST2H2BE (H2BE) HIST1H2AG (H2AG) and HIST1H3H (H3H) mRNA. Bars represent relative quantification using the ΔΔCt method normalized to control siRNA transfection +/− 95% confidence interval of three replicates. Human ACTB was used as an endogenous control.
(B) HeLa cells were treated with 1μg/mL actinomycin D or DMSO control for one hour and then crosslinked. Immunoprecipitation with antibodies against Ars2, CDK9 or a control antibody followed by Northern blotting for 7SK was performed. U1 snRNA, which binds Ars2, was probed to insure Ars2 RNA binding was not compromised by actinomycin D treatment. Detection of 7SK in Ars2 immunoprecipitates increased 5.7 fold following actinomycin D treatment while detection of U1 snRNA only increased 1.4 fold, similar to the increase in non-specific U1 binding seen in control immunoprieipitates (1.3 fold increase). Detection of 7SK in CDK9 immunoprecipitates decreased 7.1 fold following actinomycin D treatment. A shorter exposure time was used to retain linearity of 7SK signal in CDK9 immunoprecipitates (right blot). tRNATyr was probed to demonstrate the specificity of the Ars2-7SK and CDK9-7SK interactions. 100ng of total RNA was used as input.
(C) IL-3-dependent Bax−/−Bak−/− cells were grown in the presence of IL-3 until day 0, at which point media was removed, cells were washed with PBS and resuspended in media without IL-3. Cells were maintained for 14 days in IL-3 deficient media, followed by restimulation with IL-3 at day 14.5. At the time points indicated aliquots were removed from the culture and RNA was extracted for Northern blotting with a probe for 7SK. The membrane was stripped and reprobed for U6 and U11 as loading controls.
(D) IL-3-dependent Bax−/−Bak−/− cells were subjected to IL-3 withdrawal and restimulation as in (B). Aliquots of the culture were removed at the indicated time points and protein was extracted in RIPA buffer. An equal amount of total protein was run on SDS-PAGE gels and Western blotting was performed for Ars2 and CDK9.
(E) HeLa cells were transfected with control, Ars2 or 7SK siRNAs. Two days later cells were washed with PBS and fresh media was added containing 10% serum or 0.1% serum. Twenty-four hours later RNA was extracted, reverse transcribed with oligo(dT) primers and TaqMan®-based qPCR was performed to determine relative levels of polyadenylated HIST2H2BE and HIST3H2A. Bars represent relative quantification using the ΔΔCt method normalized to control siRNA transfection +/− 95% confidence interval of three replicates. Human ACTB was used as an endogenous control.
(F) Hela cells were co-transfected with siRNAs targeting Ars2 or CDK9 plus siRNAs targeting 7SK or control siRNA. Three days later RNA was extracted, reverse transcribed with oligo(dT) primers and TaqMan®-based qPCR was performed to determine relative levels of polyadenylated HIST2H2BE and HIST1H2AG. Bars represent relative quantification using the ΔΔCt method normalized to control siRNA transfection +/− 95% confidence interval of three replicates. Human ACTB was used as an endogenous control.