Abstract
Performance on measures of cognitive processing speed (CPS) slows with age, but the biological basis associated with this cognitive phenomenon remains incompletely understood. We assessed the hypothesis that the age-related slowing in CPS is associated with myelin breakdown in late-myelinating regions in a very healthy elderly population. An in vivo MRI biomarker of myelin integrity was obtained from the prefrontal lobe white matter and the genu of the corpus callosum for 152 healthy elderly adults. These regions myelinate later in brain development and are more vulnerable to breakdown due to the effects of normal aging. To evaluate regional specificity, we also assessed the splenium of the corpus callosum as a comparison region, which myelinates early in development and primarily contains axons involved in visual processing. The measure of myelin integrity was significantly correlated with CPS in highly vulnerable late-myelinating regions but not in the splenium. These results have implications for the neurobiology of the cognitive changes associated with brain aging.
Keywords: Healthy Aging, Cognition, Information Processing Speed, Myelin, White Matter, Magnetic Resonance Imaging, Alzheimer’s Disease, Dementia
INTRODUCTION
Performance on a wide-range of neuropsychological tests changes with age; for this reason, raw performance scores are typically adjusted for age to guide neuropsychological inference. In other words, the scores obtained are compared to an age-matched normative database. However, the biological basis for the age-related variance in different cognitive abilities is still not completely understood. Cognitive processing speed (CPS) is broadly defined as how fast one can execute the mental operations needed to complete a task at hand (Salthouse, 2000). Salthouse and others have argued that the age-related slowing in CPS underlies declines in other higher-order cognitive functions including memory and executive functioning (Hedden, Lautenschlager, & Park, 2005; Levitt, Fugelsang, & Crossley, 2006; Rabbitt et al., 2007; Salthouse, 1995; Salthouse, 1996; Salthouse, 2005; Salthouse & Coon, 1993; Salthouse & Ferrer-Caja, 2003).
Slowing in CPS with advancing age has high face validity and is arguably the most often replicated finding across studies of age effects on neuropsychological test performance. In clinical and laboratory settings, CPS is primarily measured using perceptual speed tasks, involving visual search, elementary comparison, and substitution operations. Cross-sectional (Gottsdanker, 1982; Salthouse, 2000; Salthouse, 2009; Tombaugh, 2004; Wilkinson & Allison, 1989) and longitudinal (Schaie, 2005) studies show that across the lifespan, speed of performance on these tasks has a quadratic trajectory that takes the shape of an inverted U; it reaches maximum efficiency around the mid-30’s then shows a generally linear decline thereafter. Recent studies have increasingly focused on the role of white matter as the biological basis underlying this basic cognitive phenomenon (Bartzokis et al., 2007; Bucur et al., 2008; Charlton et al., 2006; Deary et al., 2006; Kemper, 1994; Kennedy & Raz, 2009; Madden et al., 2009; Marner, Nyengaard, Tang, & Pakkenberg, 2003; O’Sullivan et al., 2001; Tang, Nyengaard, Pakkenberg, & Gundersen, 1997; Tuch et al., 2005; Turken et al., 2008; Vernooij et al., 2009).
Axon myelination results in saltatory conduction of action potentials (AP) that increases signal transmission speed by more than 10-fold (Waxman, 1977). Myelination also markedly decreases the refractory time (time needed for repolarization before a new AP can be supported by the axon) by as much as 34-fold (Felts, Baker, & Smith, 1997; Sinha, Karimi-Abdolrezaee, Velumian, & Fehlings, 2006). Thus, intact myelin enhances the integration of information across spatially distributed neural networks supporting cognitive and motor functions (Bartzokis et al., 2001; Fuster, 1999; Lutz, Koeneke, Wustenberg, & Jancke, 2005; Mesulam, 2000; Srinivasan, 1999). The protracted myelination process of the human brain results in a quadratic (inverted U) trajectory of myelin content and integrity as it peaks in the mid-30’s followed by breakdown and loss with advancing age (Bartzokis et al., 2001; Bartzokis et al., 2003; Benes, Turtle, Khan, & Farol, 1994; Ge et al., 2002; Jernigan & Gamst, 2005; Kemper, 1994; Walhovd et al., 2005). This course of myelin development and breakdown resembles the pattern of performance on CPS measures across the lifespan, suggesting a relationship between these brain and cognitive measures (Salthouse, 2000; Salthouse, 2009; Schaie, 2005). Furthermore, slowed information processing speed has been consistently documented in multiple sclerosis (MS), a demyelinating disease that affects the central nervous system (Archibald & Fisk, 2000; Diamond, Johnson, Kaufman, & Graves, 2008; Kail, 1998; Lengenfelder et al., 2006; Litvan, Grafman, Vendrell, & Martinez, 1998).
Myelin breakdown associated with healthy aging has been thoroughly demonstrated in animal models (Kemper, 1994; Marner et al., 2003; Peters et al., 1996; Peters & Sethares, 2003; Peters & Sethares, 2004; Peters, Sethares, & Killiany, 2001; Sloane, Hinman, Lubonia, Hollander, & Abraham, 2003; Tang et al., 1997) and humans (Pakkenberg et al., 2003). Studies using ultrastructural electron microscope reveal that age-related myelin breakdown results in microvacuolations consisting of splits in the myelin sheath layers (Kemper, 1994; Nielsen & Peters, 2000; Peters & Sethares, 2002). This creates microscopic fluid-filled spaces that increase tissue water (Bartzokis et al., 2004; Peters et al., 1996). Consequently, the structural integrity of myelin sheaths can be indirectly measured in vivo with magnetic resonance imaging (MRI) by estimating the transverse relaxation rates (R2, derived from the reciprocal of transverse relaxation time or T2). Intuitively, R2 measures how rapidly the MRI signal dissipates, and this rate is sensitive to the local molecular environment. Relaxometry measures, such as R2, are markedly sensitive to small changes in the proportion of tissue water (Oldendorf & Oldendorf, 1988). Myelination reduces white matter water content (Ferrie et al., 1999; Paus et al., 2001) and thereby increases R2; conversely, myelin breakdown increases tissue water and decreases R2 (Fazekas, Schmidt, & Scheltens, 1998; Takao et al., 1999; Dyakin et al., 2010). Histopathologic studies on animals have shown that certain toxins, such as vigabatrin (VBG), cause intramyelinic edema resulting in microvacuolations (Jackson et al., 1994; Peters et al., 1996; Peyster et al., 1995; Weiss et al., 1994); this damage is reversible with discontinuation of VGB treatment. MRI T2 signal intensity changes in concert with the extent of myelin microvacuolation, confirming that toxin-induced myelin breakdown and the subsequent recovery process can be detected and tracked with MRI (Jackson et al., 1994; Peyster et al., 1995; Qiao et al., 2000; Weiss et al., 1994) (reviewed in Cohen, Fisher, Brigell, Peyster, & Sze, 2000).
R2 measures have also been used to assess myelin integrity in humans during the development/myelination phase (birth to mid life) when R2 increases (Miot-Noirault, Barantin, Akoka, & Le Pape, 1997; Bartzokis et al., 2003) as well as in aging and a variety of myelin-damaging conditions in which R2 decreases (Takao et al., 1999; Bartzokis et al., 2003; House et al., 2006; Neema et al., 2007; Vermathen et al., 2007). Severity of myelin damage and associated R2 changes are on a continuum that ranges from focal lesions (Takao et al., 1999; Neema et al., 2007; Vermathen et al., 2007) visible to the unaided eye (referred to as T2 “hyperintensities” on radiology reports) to diffuse changes that occur in “normal appearing white matter” detectable only with quantitative R2 measures (Bartzokis et al., 2003; House et al., 2006; Neema et al., 2007; Vermathen et al., 2007). In disease processes such as multiple sclerosis or phenolketonuria, myelin destruction is qualitatively observable on MRI images but more subtle changes are also detectable quantitatively with R2 measures in “normal appearing white matter” (Neema et al., 2007; Vermathen et al., 2007). Similarly, age-related R2 changes in normal appearing white matter have been quantitatively demonstrated in healthy aging as well as more pronounced changes associated with genes that increase risk of developing Alzheimer’s disease (AD), pre-AD conditions such as mild cognitive impairment, and AD itself (Bartzokis et al., 2003; House et al., 2006; Bartzokis et al., 2007). Herein the terms myelin “integrity” and “breakdown” will be used to refer to R2 measures.
Recent studies have reported on the correlation between age and white matter integrity using diffusion tensor imaging (DTI) markers (Bucur et al., 2008; Charlton et al., 2006; Deary et al., 2006; Kennedy & Raz, 2009; Madden et al., 2009; O’Sullivan et al., 2001; Turken et al., 2008; Vernooij et al., 2009). DTI is a MRI modality that provides information about the microstructural integrity of WM by measuring the magnitude and direction of water diffusion (Pierpaoli & Basser, 1996; Basser & Pierpaoli, 1996). Fractional anisotropy (FA) describes the directional selectivity of the random motion of water molecules within a tissue and is thought to reflect the structure of axonal cell membranes and myelin sheaths (Pierpaoli & Basser 1996). Analysis of other DTI parameters such as directional diffusivities may provide additional information on the potential pathophysiology underlying the white matter changes. Increased radial diffusivity (perpendicular to the white matter tract) is related to myelin breakdown whereas changes in axial diffusivity (parallel to the primary fiber orientation) reflect axonal damage (Sun et al., 2006; Song et al. 2003, 2005). However, crossing fibers, such as those found in the frontal lobes, can artificially reduce FA values, the most frequently used index of white matter integrity, if they are computed from the standard single-tensor model (Zhan et al., 2009), but the R2 measure used here has been validated to be sensitive to myelin content (Bartzokis et al., 2004; Jackson et al., 1994; Peyster et al., 1995; Qiao et al., 2000; Weiss et al., 1994) and is not influenced by fiber orientation (Bartzokis et al., 2003; Bartzokis et al., 2004; House, St Pierre, Foster, Martins, & Clarnette, 2006) and therefore lends itself to precise regional hypothesis testing of structure-function relationships.
The heterogeneity in chronology of brain development may underlie the regionally specific pattern of myelin breakdown seen in aging, which appears to have a reverse trajectory from development, beginning from later-myelinating regions and progressing to earlier-myelinating regions (Bartzokis, 2004a; Braak & Braak, 1999; Flechsig, 1901; Kemper, 1994; Nielsen & Peters, 2000; Peters, Moss, & Sethares, 2000). Regions that myelinate later in brain development include the frontal lobes and the genu of the corpus callosum (which connects the prefrontal cortices of the left and right hemispheres). These regions are comprised of smaller axons and the myelin sheaths have fewer myelin lamellae (Chia, Thompson, & Moscarello, 1983). Therefore, these regions tend to be more vulnerable to breakdown by a variety of brain insults as well as the effects of normal aging (Kemper, 1994; Marner et al., 2003; Tang et al., 1997). In contrast, the splenium of the corpus callosum contains primarily sensory (visual) axons that tend to be fully and heavily myelinated in early childhood (Lamantia & Rakic, 1990; Pandya & Seltzer, 1986; Yakovlev & Lecours, 1967).
Here we examined associations between cognitive processing speed (CPS) and selected regional measures of myelin integrity. To assess the normal aging process, as far as possible, we studied a well-characterized healthy elderly sample selected to minimize risk of incipient Alzheimer’s disease (AD; e.g., under age 80, absence of family history of dementia and other risk factors). The use of the R2 measure, which is highly reliable and reproducible (Bartzokis et al., 2003; 2004), more specifically assesses myelin fiber integrity and is not limited by crossing fibers prevalent in regions such as the frontal lobes. We specifically chose to analyze frontal lobe white matter (FLwm) and genu of the corpus callosum (Gwm) and combine them into a single measure because they both represent late-myelinating white matter (LMwm) and are most vulnerable and maximally affected by the aging process. For a contrasting region, we analyzed the splenium (Swm), which is heavily myelinated and more resistant to age-related breakdown. The CPS tasks chosen for this study (Digit Symbol and Trails A) use a broad range of cognitive, perceptual, and motor processes and are sensitive to the integrity of late-myelinating fiber systems that are responsible for speed and efficiency of task execution (Turken et al., 2008); therefore, we would expect CPS to be associated with myelin integrity in LMwm region but not in the Swm.
METHODS
Participants
Normal adult volunteers were between the ages of 55 to 80. Age 80 was chosen as the upper age limit as the risk of developing AD peaks at this age for carriers of the apolipoprotein E (ApoE) ε4 allele (Raber, Huang, & Ashford, 2004). They were recruited from the community and hospital staff for a study of healthy aging. Subjects were excluded if they had a history of neurological disorder, psychiatric illness (including drug or alcohol abuse), or head injury resulting in loss of consciousness for more than 10 minutes. Additional exclusion criteria aimed at reducing risk of underlying AD pathology included family history of AD or other neurodegenerative disorders and failed glucose tolerance test. The subjects were physically very healthy and were excluded if they were obese (defined as body mass index [BMI] of > 30kg/m2), or if they had a history of diabetes, hypertension, or cardiovascular disease. The participants were independently functioning and had no complaints or evidence of neurocognitive impairment or gross neurological abnormalities on clinical interview and brief neurological examination (GB). Analyses were based on a total of 152 individuals with mean age of 67.0 (sd=5.9) and a mean education level of 15.5 years (sd=2.6). There were 62 men and 90 women in the sample and their racial composition was comprised of 113 Caucasian (74%), 25 Asian (16%), 12 African-American (8%), and 2 Hispanic (1%) subjects.
All subjects received written and oral information about the study and signed written informed consents approved by the local institutional review board prior to study participation. Neurocognitive measures
Trailmaking Test - Part A (Reitan & Wolfson, 1985)
Part A of the Trailmaking Test (Trails A) assesses speed of visual scanning, information processing, and graphomotor tracking. Subjects are required to rapidly connect twenty-five consecutively numbered circles. Time to complete the task serves as the variable of interest.
Digit Symbol subtest from the WAIS-R (Wechsler, 1981)
This test involves rapid copying of symbols and integrates several cognitive processes including psychomotor speed, visual scanning, simple constructional ability, and short-term memory. The score reflects the number of symbols copied after 90 seconds.
Cognitive Processing Speed (CPS)
The dependent variable examined in all the analyses was a composite measure of cognitive processing speed computed by standardizing the scores from Trails A [log transformed due to positive skew then multiplied by -1 because lower time indicates faster performance] and Digit Symbol, using the means and standard deviations from the present healthy adult sample, then averaging the z-scores.
MRI protocol
All subjects were scanned using the same 1.5 Tesla MR instrument (Picker Instruments, Cleveland, Ohio), and all scans used the same imaging protocol. Details of the protocol have been published previously (Bartzokis et al., 2003; Bartzokis et al., 1994; Bartzokis et al., 2004) and are only summarized here. Two pilot sequences were obtained to specify the location and spatial orientation of the head and the position of the axial image acquisition grid. The axial image acquisition sequence acquired interleaved contiguous slices using a Carr Purcell Meiboom Gill dual spin-echo sequence TR/TE/NEX=2500/20,90/2, 3 mm slice thickness, 256×192 acquisition matrix, and 25 cm field of view.
Image analysis
Transverse relaxation time (T2) was calculated at each voxel by an automated algorithm from the two signal intensities (TE = 20 & 90) of the robust dual spin-echo sequence that used 90 degree refocusing pulses to produce gray-scale encoded T2 maps of the brain (Bartzokis et al., 1994). T2 measures were extracted using a Macintosh configured image analysis workstation. The image analysis software (Medvision 1.4, Evergreen Technologies, Castine, ME) permitted the rater to delineate the region-of-interest (ROI) using a mouse.
For all three regions, two contiguous slices were chosen for analysis. To analyze the frontal lobe white matter, a circular ROI sample of supraorbital white matter was placed manually by the rater in the frontal lobe white matter on the second and third contiguous slices above the last image containing orbitofrontal cortex (Bartzokis et al., 2003). For analysis of the genu of the corpus callosum, the two slices on which the angle formed by the left and right sides of the genu appeared the most linear were chosen. This was done to obtain a sample that would be consistently in the middle of the structure, which contains primarily fibers connecting the prefrontal cortices (Bartzokis, Sultzer, et al., 2004). Values from these two regions formed the late myelinating white matter measure (LMwm) (Figure 1). For the contrasting early-myelinating region, the lower half of the splenium of the corpus callosum (Swm) was chosen. The second and third lowest slices on which the fibers of the splenium connected in the midline were chosen in order to sample primarily the lower half of the splenium that contains predominantly early-myelinating primary sensory (visual) fibers (Lamantia & Rakic, 1990; Pandya & Seltzer, 1986) (Figure 1).
Figure 1.
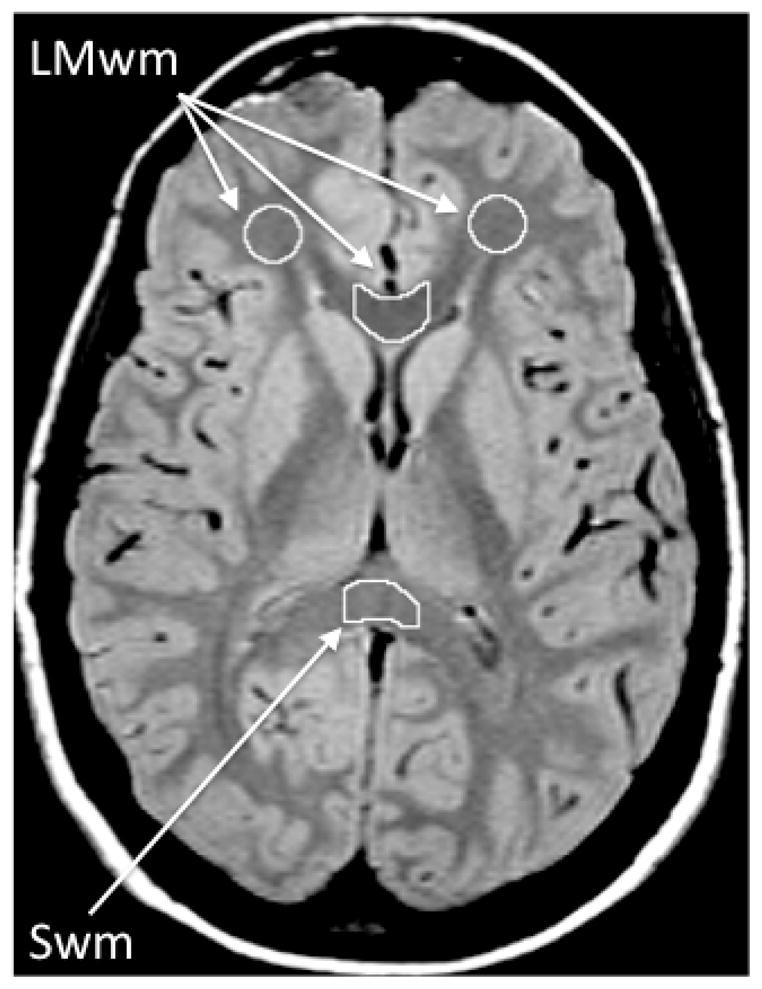
White matter regions of interest (ROI). The ROIs are depicted on an early echo (TE=20) axial MRI image that has good contrast between gray matter (light gray) and white matter (dark gray). The TE=90 (not shown) has optimal contrast between brain (appears gray) and CSF (white). Both TE=20 and TE=90 images are used to draw each ROI as this combination of slices maximizes contrast needed for optimal ROI definition. Frontal lobe white matter: The ROI is manually edited to exclude any hyperintensities or gray matter. Genu and splenium for the corpus callosum: For each of the two corpus callosum regions, a standard rectangular ROI template is first positioned on the midline, and then the anterior and posterior borders are manually edited using the contrast provided by the TE=20 and TE=90 images to exclude non-corpus callosum tissue. Lateral borders are defined by the dimensions of the rectangular template. For the genu, this positioning results in a sample consistently in the middle of the structure, which contains primarily fibers connecting the prefrontal cortices. For the splenium, this positioning samples primarily the lower half of the structure, which contains predominantly primary sensory (visual) fibers. This subject’s image was chosen as an example because the head positioning was such that frontal lobe, genu, and splenium white matter were measured on the same slices. For the majority of subjects; however, these regions are measured on different slices.
LMwm = Late-myelinating white matter (average of frontal lobe and genu of corpus callosum white matter). Swm = Splenium of corpus callosum white matter (early-myelinating region).
Once the choice of slices and position of the ROIs were completed, the rater excluded gray matter regions of the central sulcus, T2 hyperintensities, or other hyperintense structures such as periventricular halos (for further details please see Bartzokis, et al., 2003; Bartzokis, Sultzer, et al., 2004). While the general image analysis protocol describes the removal of T2 hyperintensities or other hyperintense structures if present, the white matter ROIs were not affected by T2 hyperintensities in this sample of very healthy older adults who were screened and excluded for vascular-related risk factors such as hypertension, diabetes, and cardiovascular disease. The ROIs were then transferred onto the corresponding T2 maps. All voxels that had a T2 value above the right side inflection point of the histogram of the ROI were removed, to eliminate voxels that partially contained CSF structures or lesions (Bartzokis, et al., 1994).
T2 data for each ROI were obtained from contiguous pairs of slices. The relaxation rate (R2) was calculated as the reciprocal of T2, and then multiplied by 1000 (by convention). The average R2 of the two slices from both hemispheres were the final measures used in the subsequent analyses. Test-retest reliability of the R2 measurement was assessed by computing intraclass correlation coefficients on 2 ratings done at least 1 month apart by the same rater for 13 of the scans. Reliability was very good for all three regions (Frontal lobe white matter: Rxx=0.91, F=21.3, df=1,12, p<.0001; genu of the corpus callosum white matter: Rxx=0.99, F=138.0, df=1,11, p<.0001; splenium of the corpus callosum white matter: Rxx=0.95, F=20.5, df=1,11, p<.0001).
Data Analysis
The primary hypothesis was that there would be a significant relationship between CPS and R2 in late-myelinating regions (LMwm; composed of the average R2 for the frontal and genu of the corpus callosum white matter regions) but not in the early-myelinating region (Swm). We compared age-related linear change in R2 in late- and early-myelinating regions using multivariate multiple regression with age as the independent variable, testing the difference in the regression coefficients for the R2 measure in the two regions. A simple linear regression model was used to examine age-related change in the measure of CPS. To complement the regression analysis, Pearson correlations were computed separately between measures of R2 in the two regions and age, CPS and age, and between R2 and CPS. Differences between these correlations were tested using a normal curve test based on Fisher’s z-transformation. Significance tests are unadjusted for multiple comparisons and are reported as significant at p=.05 (two-tailed).
RESULTS
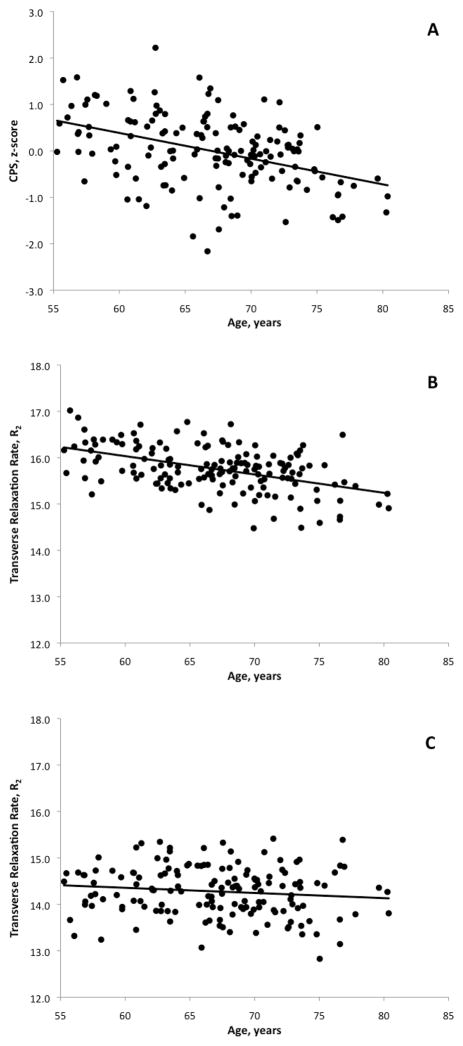
In this sample, the slope of the associations between measures of myelin integrity (R2) and age were negative for both regions, but the slope of R2 with age was steeper for LMwm than in Swm (LMwm: −.040 per year, SE=.006, p<.0001; Swm: −.011 per year, SE=.007, p=.133; multivariate F=14.92, df=1, 150, p=.0002). A steep slope was also seen for the association between CPS and age (−.055 per year, SE=.010, p<.0001). We computed Pearson correlations which revealed a significant negative relationship between LMwm and age (r=−.473, df=150, p<.0001) but not between Swm and age (r=−.123, df=150, p=.133). The difference in correlation coefficients between the LMwm and Swm groups were statistically significant and mirrored the regression result (Fisher’s z-test: t=4.49, df=149, p<.0001). Significant negative relationship was also observed between CPS and age (r=−.423, df=150, p<.0001). These associations are graphically displayed in Figures 2A–C.
Figure 2.
Figures 2A–2C. Scatterplots and age-regression lines of cognitive processing speed (CPS)(A) late-myelinating white matter (B), and splenium of the corpus callosum (C).
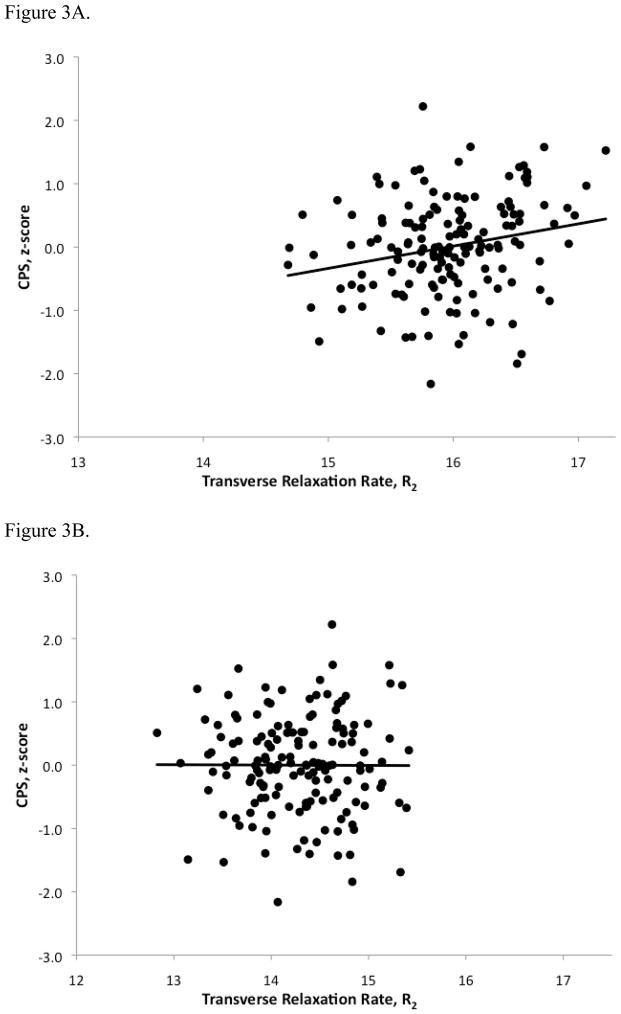
Pearson correlation analyses showed that CPS was significantly associated with R2 in the LMwm (r=.225, df=150, p=.005) but not Swm (r=−.004, df=150, p=.961). Correlation coefficients between the two regions were compared via z-transformation, and the difference was statistically significant (t=2.66, df=149, p=.009) indicating a significant regional difference in the association between CPS and R2. The relationship between LMwm R2 and CPS was no longer statistically significant after adjusting for the effects of age using partial correlation analysis (r=.031, df=149 p=.702).
Figure 3A shows the scatterplot and the trend line of the hypothesized association between LMwm R2 and CPS for the healthy adult group, while Figure 3B shows the absence of this association in the contrasting region of Swm.
Figure 3.
Figures 3A and 3B. Scatterplots of Cognitive Processing Speed (CPS) versus Transverse Relaxation Rate (R2) in late-myelinating white matter (3A) and splenium of the corpus callosum (3B) of healthy elderly subjects.
DISCUSSION
Our data confirm prior observations that CPS slows significantly with age (Gottsdanker, 1982; Salthouse, 2000; Salthouse, 2009, Tombaugh, 2004; Schaie, 2005; Wilkinson & Allison, 1989) and that LMwm was more susceptible to age-related myelin breakdown than the earlier-myelinating Swm region (Bartzokis, Sultzer, et al., 2004; House et al., 2006). As hypothesized, the R2 was correlated with CPS only in the late-myelinating region, confirming regional specificity in this structure-function relationship. The relationship between LMwm R2 and CPS was no longer statistically significant after adjusting for age indicating that both measures are strongly affected by the aging process.
By middle age, the developmental process of myelination produces a continuum of increasingly vulnerable oligodendrocytes from less vulnerable early-myelinating regions such as the visual pathways to more vulnerable later-myelinating association brain regions such as the frontal lobes (Bartzokis, Sultzer, et al., 2004; Flechsig, 1901; Meyer, 1981). Later-differentiating oligodendrocytes ensheath increasing numbers of axons with smaller axon diameters (Lamantia & Rakic, 1990; Pandya & Seltzer, 1986). As a result of increased complexity and metabolic demands, these more vulnerable myelin sheaths are disproportionately lost with age (27–45% reduction) (Bartzokis, Sultzer, et al., 2004; Braak & Braak, 1991; Kemper, 1994; Marner et al., 2003; Tang et al., 1997). The uniquely extensive myelination of the human brain makes myelin maintenance critical for sustaining our high CPS. The data support the hypothesis that during normal aging, the myelin-breakdown process in highly vulnerable late-myelinating regions is associated with a degradation of CPS (Bartzokis, 2004a, 2005, 2009).
Present findings provide a potential biological substrate for the “frontal aging hypothesis,” which proposes that the frontal lobes, a late myelinating region, are most vulnerable to age-related deterioration and thus underlie the neuropsychology of aging (Dempster, 1992; Greenwood, 2000). However, “frontal aging” may be preceded and mediated by the more basic cognitive phenomenon of processing speed as Salthouse and others have argued that the age-related slowing in CPS underlies declines in other higher-order cognitive functions such as memory and executive functioning (Hedden et al., 2005; Levitt et al., 2006; Rabbitt et al., 2007; Salthouse, 1995; Salthouse, 1996; Salthouse, 2005; Salthouse & Coon, 1993; Salthouse & Ferrer-Caja, 2003). This is consistent with large longitudinal studies of cognitive performance across the lifespan, which show that the decline in CPS performance begins at an earlier age and progresses at a steeper rate compared to memory functioning, which declines later in life (Amieva, Rouch-Leroyer, Letenneur, Dartigues, & Fabrigoule, 2004; Schaie, 2005). The CPS-R2 associations observed in the present study, when considered in conjunction with the DTI literature demonstrating that integrity of specific white matter tracts can mediate age-related slowing in cognitive processing speed (Madden et al., 2009; Bucur et al., 2008; Gold et al., 2010), support the proposition that myelin breakdown may represent one biological source of age-related decline in cognition. This has also been shown in non-human primates (Peters et al., 2000; Peters & Sethares, 2002), and explains the need for age adjustments in determining “normal” neuropsychological performance.
Studies relating white matter microstructure to cognition have received increased attention in recent years, but our current study represents a unique contribution to the existing literature because 1) our strict entry criteria attempt to create a model that best represents healthy brain and cognitive aging in humans, 2) the use of the R2 measure more specifically assesses myelin fiber integrity as opposed to general white matter or axon health indicated by DTI markers employed by the majority of studies (Vernooij et al., 2009). To our knowledge, this is the only imaging study on healthy aging that aggressively screened out risks of incipient AD (e.g., no family history of AD, under age 80, no subjective concerns or objectively documented cognitive problems, normal glucose tolerance testing, no head trauma, excellent physical health, etc.). This minimizes the possibility that age-related changes in cognition and myelin integrity are driven by AD pathology. This study design replicates myelin-cognition associations observed post mortem in non-human primates, which do not develop AD (Peters et al., 2000; Peters & Sethares, 2002).
Recent studies have reported similar results using DTI markers, namely significant relationships between FA of anterior brain regions and tests of cognitive functions, including measures of information processing speed (Bucur et al., 2008; Charlton et al., 2006; Kennedy & Raz, 2009; Stebbins et al., 2001; Turken et al., 2008; Vernooij et al., 2009) and executive functioning involving attentional set-shifting and working memory (Charlton et al., 2006; Kennedy & Raz, 2009; O’Sullivan et al., 2001). In addition, lower FA in the anterior limb of the internal capsule was found to be associated with slower reaction time in older individuals (Madden et al., 2004) while FA in the splenium and parietal pericallosal region significantly correlated with alternated finger tapping (Sullivan et al., 2001). We did not find a significant association between splenium R2 and finger tapping speed (Bartzokis et al., 2010) or the CPS measures in the present study. The discrepant results may reflect differential sensitivity of R2 and FA measures to aspects of splenium cellular structure that correlate with finger tapping. R2 and DTI, depending on the indices used and regions examined, may demonstrate similar or different patterns of development and breakdown with age. Specifically, R2 of frontal lobes shows a quadratic, inverted-U trajectory (Bartzokis et al., 2003) consistent with post mortem data (Benes et al., 1994; Kemper, 1994). While several studies have reported that FA in the same region declines approximately linearly with age beginning from early adulthood (Grieve, Williams, Paul, Clark, & Gordon, 2007; Salat et al., 2005; Hsu et al. 2010), recent literature has demonstrated non-linear, quadratic relationships between FA and age in specific regions such as the body of the corpus callosum (Hsu et al., 2010; Sala et al., 2010), limbic pathways, and association and corticospinal tracts (Sala et al., 2010). In summary, R2 and DTI offer different methods for assessing myelin content/white matter integrity, but the two approaches are complementary and their combined use will likely yield greater insight into the underlying pathophysiology of white matter changes.
Several study limitations should be acknowledged. The selection of very healthy individuals and the exclusion of family history with dementia may limit potential confounds and contributors that affect the trajectory of brain and cognitive changes, but the present findings are likely to underestimate the R2-CPS relationship compared to the general population. A number of tissue changes such as subtle edema, alteration in regional vasculature, or alterations of axons unrelated to myelin breakdown could result in small changes in regional water content, affecting CPS and R2. This possibility is less likely as all subjects had been thoroughly assessed and found to be in excellent health, and had few risk factors for vascular disease or significant history of head trauma. The measures that comprise the CPS all involve graphomotor demands, thus performance may be adversely impacted by physical limitations and ailments (e.g., arthritis); subsequent studies may choose to employ cognitive processing speed measures devoid of a motor component. Finally, in a cross-sectional study, interpretation of age-related differences as “changes” or as “cause and effect’ should be avoided (Kraemer, Yesavage, Taylor, & Kupfer, 2000; Schaie, 2005). Although more time-consuming and costly, prospective studies may be advantageous to more accurately model trajectories of change.
There is an urgent need to understand the biological and functional changes associated with brain aging, as age is the most potent risk factor for developing AD. The ability to measure age-related breakdown of myelin in vivo and the associated decline in CPS provides a biological framework to interpret changes in cognition associated with brain aging. Furthermore, measures of myelin integrity hold promise as a possible surrogate biomarker of assessing cognitive decline and outcome for primary prevention trials. Future studies can extend the present findings to examine the contribution of myelin breakdown to the trajectory of decline from normal cognitive functioning into MCI and AD.
Acknowledgments
This work was supported by grant K23-AG028727 from the National Institute of Aging (NIA), a grant from the Alzheimer’s Association (NIRG-07-60424), NIH grants (MH 0266029; AG027342), the Alzheimer’s Disease Research Center grant P50 AG-16570, and the California Alzheimer’s Disease Centers of California. Additional support for the study came from the RCS Alzheimer’s Foundation and the Department of Veterans Affairs.
Footnotes
Disclosures: The authors have nothing to disclose
References
- Amieva H, Rouch-Leroyer I, Letenneur L, Dartigues JF, Fabrigoule C. Cognitive slowing and learning of target detection skills in pre-demented subjects. Brain Cogn. 2004;54(3):212–214. doi: 10.1016/j.bandc.2004.02.003. [DOI] [PubMed] [Google Scholar]
- Archibald CJ, Fisk JD. Information processing efficiency in patients with multiple sclerosis. Journal of Clinical and Experimental Neuropsychology. 2000;22:686–701. doi: 10.1076/1380-3395(200010)22:5;1-9;FT686. [DOI] [PubMed] [Google Scholar]
- Bartzokis G. Age-related myelin breakdown: a developmental model of cognitive decline and Alzheimer’s disease. Neurobiol Aging. 2004a;25(1):5–18. doi: 10.1016/j.neurobiolaging.2003.03.001. [DOI] [PubMed] [Google Scholar]
- Bartzokis G. Quadratic trajectories of brain myelin content: unifying construct for neuropsychiatric disorders. Neurobiol Aging. 2004b;25(1):49–62. [Google Scholar]
- Bartzokis G. Brain myelination in prevalent neuropsychiatric developmental disorders: Primary and comorbid addiction. Adolescent Psychiatry. 2005;29:55–96. [PMC free article] [PubMed] [Google Scholar]
- Bartzokis G. Alzheimer’s disease as Homeostatic Responses to Age-Related Myelin Breakdown. Neurobiology of Aging. 2009 doi: 10.1016/j.neurobiolaging.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartzokis G, Beckson M, Lu PH, Nuechterlein KH, Edwards N, Mintz J. Age-related changes in frontal and temporal lobe volumes in men: A magnetic resonance imaging study. Arch Gen Psychiatry. 2001;58:461–465. doi: 10.1001/archpsyc.58.5.461. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Cummings JL, Sultzer D, Henderson VW, Nuechterlein KH, Mintz J. White matter structural integrity in healthy aging adults and patients with Alzheimer disease: a magnetic resonance imaging study. Arch Neurol. 2003;60(3):393–398. doi: 10.1001/archneur.60.3.393. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Lu PH, Geschwind D, Tingus K, Huang D, Mendez MF, et al. Apolipoprotein E Affects Both Myelin Breakdown and Cognition: Implications for Age-Related Trajectories of Decline Into Dementia. Biological Psychiatry. 2007;62(12):1380–1387. doi: 10.1016/j.biopsych.2007.03.024. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Lu PH, Mintz J. Quantifying age-related myelin breakdown with MRI: Novel therapeutic targets for preventing cognitive decline and Alzheimer’s disease. J Alzheimers Dis. 2004;6:S53–59. doi: 10.3233/jad-2004-6s604. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Mintz J, Sultzer D, Marx P, Herzberg JS, Phelan CK, et al. In vivo MR evaluation of age-related increases in brain iron. AJNR Am J Neuroradiol. 1994;15:1129–1138. [PMC free article] [PubMed] [Google Scholar]
- Bartzokis G, Sultzer D, Lu PH, Nuechterlein KH, Mintz J, Cummings J. Heterogeneous age-related breakdown of white matter structural integrity: Implications for cortical “disconnection” in aging and Alzheimer’s disease. Neurobiol Aging. 2004;25(7):843–851. doi: 10.1016/j.neurobiolaging.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J Magn Reson B. 1996;111(3):209–219. doi: 10.1006/jmrb.1996.0086. [DOI] [PubMed] [Google Scholar]
- Benedict C, Hallschmid M, Hatke A, Schultes B, Fehm HL, Born J, et al. Intranasal insulin improves memory in humans. Psychoneuroendocrinology. 2004;29(10):1326–1334. doi: 10.1016/j.psyneuen.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Benes FM, Turtle M, Khan Y, Farol P. Myelination of a key relay zone in the hippocampal formation occurs in the human brain during childhood, adolescence, and adulthood. Arch Gen Psychiatry. 1994;51:477–484. doi: 10.1001/archpsyc.1994.03950060041004. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol (Berl) 1991;82(4):239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Temporal sequence of Alzheimer’s disease-related pathology. In: Jones E, Peters A, editors. Cerebral Cortex: Neurodegenerative and age-related changes in structure and function of cerebral cortex. Vol. 14. New York: Plenium Press; 1999. pp. 475–512. [Google Scholar]
- Bucur B, Madden DJ, Spaniol J, Provenzale JM, Cabeza R, White LE, et al. Age-related slowing of memory retrieval: contributions of perceptual speed and cerebral white matter integrity. Neurobiol Aging. 2008;29(7):1070–1079. doi: 10.1016/j.neurobiolaging.2007.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne D, Pearson D, Dea D, Rochford J, Poirier J. The cholesterol-lowering drug probucol increases apolipoprotein E production in the hippocampus of aged rats: implications for Alzheimer’s disease. Neuroscience. 2003;121(1):99–110. doi: 10.1016/s0306-4522(03)00361-0. [DOI] [PubMed] [Google Scholar]
- Charlton RA, Barrick TR, McIntyre DJ, Shen Y, O’Sullivan M, Howe FA, et al. White matter damage on diffusion tensor imaging correlates with age-related cognitive decline. Neurology. 2006;66(2):217–222. doi: 10.1212/01.wnl.0000194256.15247.83. [DOI] [PubMed] [Google Scholar]
- Chia LS, Thompson JE, Moscarello MA. Changes in lipid phase behaviour in human myelin during maturation and aging. FEBS. 1983;157(1):155–158. doi: 10.1016/0014-5793(83)81136-3. [DOI] [PubMed] [Google Scholar]
- Cohen JA, Fisher RS, Brigell MG, Peyster RG, Sze G. The potential for vigabatrin-induced intramyelinic edema in humans. Epilepsia. 2000;41(2):148–157. doi: 10.1111/j.1528-1157.2000.tb00134.x. [DOI] [PubMed] [Google Scholar]
- Deary IJ, Bastin ME, Pattie A, Clayden JD, Whalley LJ, Starr JM, et al. White matter integrity and cognition in childhood and old age. Neurology. 2006;66(4):505–512. doi: 10.1212/01.wnl.0000199954.81900.e2. [DOI] [PubMed] [Google Scholar]
- Dempster FN. The rise and fall of the inhibitory mechanism: Toward a unified theory of cognitive development and aging. Developmental Review. 192;12:45–75. [Google Scholar]
- Diamond BJ, Johnson SK, Kaufman M, Graves L. Relationships between information processing, depression, fatigue, and cognition in multiple sclerosis. Archives of Clinical Neuropsychology. 2008;23:189–199. doi: 10.1016/j.acn.2007.10.002. [DOI] [PubMed] [Google Scholar]
- Dyakin VV, Chen Y, Branch CA, Veeranna, Yuan A, Rao M, et al. The contributions of myelin and axonal caliber to transverse relaxation time in shiverer and neurofilament-deficient mouse models. Neuroimage. 2010;51(3):1098–1105. doi: 10.1016/j.neuroimage.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazekas F, Schmidt R, Scheltens P. Pathophysiologic mechanisms in the development of age-related white matter changes of the brain. Dement Geriatr Cogn Disord. 1998;9(Suppl 1):2–5. doi: 10.1159/000051182. [DOI] [PubMed] [Google Scholar]
- Felts PA, Baker TA, Smith KJ. Conduction in segmentally demyelinated mammalian central axons. J Neurosci. 1997;17(19):7267–7277. doi: 10.1523/JNEUROSCI.17-19-07267.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrie JC, Barantin L, Saliba E, Akoka S, Tranquart F, Sirinelli D, et al. MR assessment of the brain maturation during the perinatal period: quantitative T2 MR study in premature newborns. Magn Reson Imaging. 1999;17(9):1275–1288. doi: 10.1016/s0730-725x(99)00080-6. [DOI] [PubMed] [Google Scholar]
- Flechsig P. Developmental (Myelogenetic) Localisation of the Cerebral Cortex in the Human Subject. Lancet. 1901:1027–1029. [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Fuster JM. Synopsis of function and dysfunction of the frontal lobe. Acta Psychiatr Scand Suppl. 1999;395:51–57. doi: 10.1111/j.1600-0447.1999.tb05983.x. [DOI] [PubMed] [Google Scholar]
- Ge Y, Grossman RI, Babb JS, Rabin ML, Mannon LJ, Kolson DL. Age-Related Total Gray Matter and White Matter Changes in Normal Adult Brain. Part I: Volumetric MR Imaging Analysis. AJNR Am J Neuroradiol. 2002;23(8):1327–1333. [PMC free article] [PubMed] [Google Scholar]
- Gomez-Isla T, Hollister R, West H, Mui S, Growdon JH, Petersen RC, et al. Neuronal loss correlates with but exceeds neurofibrillary tangles in Alzheimer’s disease. Ann Neurol. 1997;41(1):17–24. doi: 10.1002/ana.410410106. [DOI] [PubMed] [Google Scholar]
- Gottsdanker R. Age and simple reaction time. J Gerontol. 1982;37(3):342–348. doi: 10.1093/geronj/37.3.342. [DOI] [PubMed] [Google Scholar]
- Greenwood PM. The frontal aging hypothesis evaluated. Journal of the International Neuropsychological Society. 2000;6:705–726. doi: 10.1017/s1355617700666092. [DOI] [PubMed] [Google Scholar]
- Grieve SM, Williams LM, Paul RH, Clark CR, Gordon E. Cognitive Aging, Executive Function, and Fractional Anisotropy: A Diffusion Tensor MR Imaging Study. AJNR Am J Neuroradiol. 2007;28(2):226–235. [PMC free article] [PubMed] [Google Scholar]
- Hara M, Matsushima T, Satoh H, Iso-o N, Noto H, Togo M, et al. Isoform-dependent cholesterol efflux from macrophages by apolipoprotein E is modulated by cell surface proteoglycans. Arterioscler Thromb Vasc Biol. 2003;23(2):269–274. doi: 10.1161/01.atv.0000054199.78458.4b. [DOI] [PubMed] [Google Scholar]
- Hedden T, Lautenschlager G, Park DC. Contributions of processing ability and knowledge to verbal memory tasks across the adult life-span. Q J Exp Psychol A. 2005;58(1):169–190. doi: 10.1080/02724980443000179. [DOI] [PubMed] [Google Scholar]
- House MJ, St Pierre TG, Foster JK, Martins RN, Clarnette R. Quantitative MR imaging R2 relaxometry in elderly participants reporting memory loss. AJNR Am J Neuroradiol. 2006;27(2):430–439. [PMC free article] [PubMed] [Google Scholar]
- Hsu JL, Van Hecke W, Bai CH, Lee CH, Tsai YF, Chiu HC, Jaw FS, Hsu CY, Leu JG, Chen WH, Leemans A. Microstructural white matter changes in normal aging: A diffusion tensor imaging study with higher-order polynomial regression models. Neuro Image. 2010;49:32–43. doi: 10.1016/j.neuroimage.2009.08.031. [DOI] [PubMed] [Google Scholar]
- Jackson GD, Williams SR, Weller RO, van Bruggen N, Preece NE, Williams SC, et al. Vigabatrin-induced lesions in the rat brain demonstrated by quantitative magnetic resonance imaging. Epilepsy Res. 1994;18(1):57–66. doi: 10.1016/0920-1211(94)90033-7. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Gamst AC. Changes in volume with age-consistency and interpretation of observed effects. Neurobiol Aging. 2005;26(9):1271–1274. doi: 10.1016/j.neurobiolaging.2005.05.016. [DOI] [PubMed] [Google Scholar]
- Kail R. Speed of information processing in patients with multiple sclerosis. Journal of Clinical and Experimental Neuropsychology. 1998;20:98–106. doi: 10.1076/jcen.20.1.98.1483. [DOI] [PubMed] [Google Scholar]
- Kemper T. Neuroanatomical and neuropathological changes during aging and dementia. In: Albert M, Knoefel J, editors. Clinical Neurology of Aging. 2. New York: Oxford University Press; 1994. pp. 3–67. [Google Scholar]
- Kennedy KM, Raz N. Aging white matter and cognition: differential effects of regional variations in diffusion properties on memory, executive functions, and speed. Neuropsychologia. 2009;47(3):916–927. doi: 10.1016/j.neuropsychologia.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraemer HC, Yesavage JA, Taylor JL, Kupfer D. How can we learn about developmental processes from cross-sectional studies, or can we? Am J Psychiatry. 2000;157(2):163–171. doi: 10.1176/appi.ajp.157.2.163. [DOI] [PubMed] [Google Scholar]
- Lamantia AS, Rakic P. Cytological and quantitative characteristics of four cerebral commissures in the rhesus monkey. J Comp Neurol. 1990;291(4):520–537. doi: 10.1002/cne.902910404. [DOI] [PubMed] [Google Scholar]
- Lengenfelder J, Bryant D, Diamond BJ, Kalmar JH, Moore NB, DeLuca J. Processing speed interacts with working memory efficiency in multiple sclerosis. Archives of Clinical Neuropsychology. 2006;21:229–238. doi: 10.1016/j.acn.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Levitt T, Fugelsang J, Crossley M. Processing speed, attentional capacity, and age-related memory change. Exp Aging Res. 2006;32(3):263–295. doi: 10.1080/03610730600699118. [DOI] [PubMed] [Google Scholar]
- Litvan I, Grafman J, Vendrell P, Martinez JM. Slowed information processing in multiple sclerosis. Archives of Neurology. 1988;34:281–285. doi: 10.1001/archneur.1988.00520270059021. [DOI] [PubMed] [Google Scholar]
- Lutz K, Koeneke S, Wustenberg T, Jancke L. Asymmetry of cortical activation during maximum and convenient tapping speed. Neurosci Lett. 2005;373(1):61–66. doi: 10.1016/j.neulet.2004.09.058. [DOI] [PubMed] [Google Scholar]
- Mabile L, Lefebvre C, Lavigne J, Boulet L, Davignon J, Lussier-Cacan S, et al. Secreted apolipoprotein E reduces macrophage-mediated LDL oxidation in an isoform-dependent way. J Cell Biochem. 2003;90(4):766–776. doi: 10.1002/jcb.10697. [DOI] [PubMed] [Google Scholar]
- Madden DJ, Spaniol J, Costello MC, Bucur B, White LE, Cabeza R, et al. Cerebral white matter integrity mediates adult age differences in cognitive performance. J Cogn Neurosci. 2009;21(2):289–302. doi: 10.1162/jocn.2009.21047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marner L, Nyengaard JR, Tang Y, Pakkenberg B. Marked loss of myelinated nerve fibers in the human brain with age. J Comp Neurol. 2003;462(2):144–152. doi: 10.1002/cne.10714. [DOI] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Mesulam M. Brain, mind, and the evolution of connectivity. Brain Cogn. 2000;42(1):4–6. doi: 10.1006/brcg.1999.1145. [DOI] [PubMed] [Google Scholar]
- Meyer A. Paul Flechsig’s system of myelogenetic cortical localization in the light of recent research in neuroanatomy and neurophysiology. Part I. Can J Neurol Sci. 1981;8(1):1–6. doi: 10.1017/s031716710004275x. [DOI] [PubMed] [Google Scholar]
- Miot-Noirault E, Barantin L, Akoka S, Le Pape A. T2 relaxation time as a marker of brain myelination: experimental MR study in two neonatal animal models. J Neurosci Methods. 1997;72:5–14. doi: 10.1016/s0165-0270(96)00148-3. [DOI] [PubMed] [Google Scholar]
- Neema M, Stankiewicz J, Arora A, Dandamudi VS, Batt CE, Guss ZD, Al-Sabbagh A, Bakshi R. T1- and T2-based MRI measures of diffuse gray matter and white matter damage in patients with multiple sclerosis. J Neuroimaging. 2007;17(Suppl 1):16S–21S. doi: 10.1111/j.1552-6569.2007.00131.x. [DOI] [PubMed] [Google Scholar]
- Nielsen K, Peters A. The effects of aging on the frequency of nerve fibers in rhesus monkey striate cortex. Neurobiol Aging. 2000;21(5):621–628. doi: 10.1016/s0197-4580(00)00169-x. [DOI] [PubMed] [Google Scholar]
- O’Sullivan M, Jones DK, Summers PE, Morris RG, Williams SCR, Markus HS. Evidence for cortical “disconnection” as a mechanism of age-related cognitive decline. Neurology. 2001;57:632–638. doi: 10.1212/wnl.57.4.632. [DOI] [PubMed] [Google Scholar]
- Oldendorf WH, Oldendorf W., Jr . Basics of Magnetic Resonance Imaging. Boston, M.A: Martinus Nijhof Publishing; 1988. [Google Scholar]
- Pakkenberg B, Pelvig D, Marner L, Bundgaard MJ, Gundersen HJG, Nyengaard JR, Regeur L. Aging and the human neocortex. Experimental Gerontology. 2003;38:95–99. doi: 10.1016/s0531-5565(02)00151-1. [DOI] [PubMed] [Google Scholar]
- Pandya DN, Seltzer B. Two Hemispheres-One Brain: Functions of the Corpus Callosum. Alan R. Liss, Inc; 1986. The topography of commissural fibers; pp. 47–73. [Google Scholar]
- Paus T, Collins DL, Evans AC, Leonard G, Pike B, Zjidenbos A. Maturation of white matter in the human brain: a review of magnetic resonance studies. Brain Research Bulletin. 2001;54(3):255–256. doi: 10.1016/s0361-9230(00)00434-2. [DOI] [PubMed] [Google Scholar]
- Peters A. Structural changes in the normally aging cerebral cortex of primates. Prog Brain Res. 2002;136:455–465. doi: 10.1016/s0079-6123(02)36038-2. [DOI] [PubMed] [Google Scholar]
- Peters A, Morrison JH, Rosene DL, Hyman BT. Are neurons lost from the primate cerebral cortex during normal aging? Cereb Cortex. 1998;8:295–300. doi: 10.1093/cercor/8.4.295. [DOI] [PubMed] [Google Scholar]
- Peters A, Moss MB, Sethares C. Effects of aging on myelinated nerve fibers in monkey primary visual cortex. J Comp Neurol. 2000;419(3):364–376. doi: 10.1002/(sici)1096-9861(20000410)419:3<364::aid-cne8>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Peters A, Rosene DL, Moss MB, Kemper TL, Abraham CR, Tigges J, et al. Neurobiological bases of age-related cognitive decline in the rhesus monkey. J Neuropathol Exp Neurol. 1996;55(8):861–874. doi: 10.1097/00005072-199608000-00001. [DOI] [PubMed] [Google Scholar]
- Peters A, Sethares C. Aging and the myelinated fibers in prefrontal cortex and corpus callosum of the monkey. J Comp Neurol. 2002;442(3):277–291. doi: 10.1002/cne.10099. [DOI] [PubMed] [Google Scholar]
- Peters A, Sethares C. Is there remyelination during aging of the primate central nervous system? J Comp Neurol. 2003;460(2):238–254. doi: 10.1002/cne.10639. [DOI] [PubMed] [Google Scholar]
- Peters A, Sethares C. Oligodendrocytes, their Progenitors and other Neuroglial Cells in the Aging Primate Cerebral Cortex. Cereb Cortex. 2004;14:995–1007. doi: 10.1093/cercor/bhh060. [DOI] [PubMed] [Google Scholar]
- Peters A, Sethares C, Killiany RJ. Effects of age on the thickness of myelin sheaths in monkey primary visual cortex. J Comp Neurol. 2001;435(2):241–248. doi: 10.1002/cne.1205. [DOI] [PubMed] [Google Scholar]
- Peyster RG, Sussman NM, Hershey BL, Heydorn WE, Meyerson LR, Yarrington JT, et al. Use of ex vivo magnetic resonance imaging to detect onset of vigabatrin-induced intramyelinic edema in canine brain. Epilepsia. 1995;36(1):93–100. doi: 10.1111/j.1528-1157.1995.tb01672.x. [DOI] [PubMed] [Google Scholar]
- Pierpaoli C, Basser PJ. Toward a Quantitative Assessment of Diffusion Anisotropy. MRM. 1996;36:893–906. doi: 10.1002/mrm.1910360612. [DOI] [PubMed] [Google Scholar]
- Qiao M, Malisza KL, Del Bigio MR, Kozlowski P, Seshia SS, Tuor UI. Effect of long-term vigabatrin administration on the immature rat brain. Epilepsia. 2000;41(6):655–665. doi: 10.1111/j.1528-1157.2000.tb00225.x. [DOI] [PubMed] [Google Scholar]
- Rabbitt P, Scott M, Lunn M, Thacker N, Lowe C, Pendleton N, et al. White matter lesions account for all age-related declines in speed but not in intelligence. Neuropsychology. 2007;21(3):363–370. doi: 10.1037/0894-4105.21.3.363. [DOI] [PubMed] [Google Scholar]
- Raber J, Huang Y, Ashford JW. ApoE genotype accounts for the vast majority of AD risk and AD pathology. Neurobiol Aging. 2004;25(5):641–650. doi: 10.1016/j.neurobiolaging.2003.12.023. [DOI] [PubMed] [Google Scholar]
- Reitan R, Wolfson D. The Halstead-Reitan Neuropsychological Test Battery. Tucson, AZ: Neuropsychology Press; 1985. [Google Scholar]
- Sadowski M, Pankiewicz J, Scholtzova H, Ripellino JA, Li Y, Schmidt SD, et al. A synthetic peptide blocking the apolipoprotein E/beta-amyloid binding mitigates beta-amyloid toxicity and fibril formation in vitro and reduces beta-amyloid plaques in transgenic mice. Am J Pathol. 2004;165(3):937–948. doi: 10.1016/s0002-9440(10)63355-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sala S, Agosta F, Pagani E, Copetti M, Comi G, Filippi M. Microstructural changes and atrophy in brain white matter tracts with aging. Neurobiology of Aging. 2010 doi: 10.1016/j.neurobiolaging.2010.04.027. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Salat DH, Tuch DS, Greve DN, van der Kouwe AJ, Hevelone ND, Zaleta AK, et al. Age-related alterations in white matter microstructure measured by diffusion tensor imaging. Neurobiol Aging. 2005;26(8):1215–1227. doi: 10.1016/j.neurobiolaging.2004.09.017. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. Selective influences of age and speed on associative memory. Am J Psychol. 1995;108(3):381–396. [PubMed] [Google Scholar]
- Salthouse TA. The processing-speed theory of adult age differences in cognition. Psychological Review. 1996;103(3):403–428. doi: 10.1037/0033-295x.103.3.403. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. Aging and measures of processing speed. Biol Psychol. 2000;54(1–3):35–54. doi: 10.1016/s0301-0511(00)00052-1. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. Relations between cognitive abilities and measures of executive functioning. Neuropsychology. 2005;19(4):532–545. doi: 10.1037/0894-4105.19.4.532. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. When does age-related cognitive decline begin? Neurobiol Aging. 2009 doi: 10.1016/j.neurobiolaging.2008.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA, Coon VE. Influence of task-specific processing speed on age differences in memory. J Gerontol. 1993;48(5):P245–255. doi: 10.1093/geronj/48.5.p245. [DOI] [PubMed] [Google Scholar]
- Salthouse TA, Ferrer-Caja E. What needs to be explained to account for age-related effects on multiple cognitive variables? Psychol Aging. 2003;18(1):91–110. doi: 10.1037/0882-7974.18.1.91. [DOI] [PubMed] [Google Scholar]
- Schaie KW. What Can We Learn From Longitudinal Studies of Adult Development? Res Hum Dev. 2005;2(3):133–158. doi: 10.1207/s15427617rhd0203_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha K, Karimi-Abdolrezaee S, Velumian AA, Fehlings MG. Functional changes in genetically dysmyelinated spinal cord axons of shiverer mice: role of juxtaparanodal Kv1 family K+ channels. J Neurophysiol. 2006;95(3):1683–1695. doi: 10.1152/jn.00899.2005. [DOI] [PubMed] [Google Scholar]
- Sloane JA, Hinman JD, Lubonia M, Hollander W, Abraham CR. Age-dependent myelin degeneration and proteolysis of oligodendrocyte proteins is associated with the activation of calpain-1 in the rhesus monkey. J Neurochem. 2003;84(1):157–168. doi: 10.1046/j.1471-4159.2003.01541.x. [DOI] [PubMed] [Google Scholar]
- Song SK, Sun SW, Ju WK, Lin SJ, Cross AH, Neufeld AH. Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. Neuroimage. 2003;20(3):1714–1722. doi: 10.1016/j.neuroimage.2003.07.005. [DOI] [PubMed] [Google Scholar]
- Song SK, Yoshino J, Le TQ, Lin SJ, Sun SW, Cross AH, et al. Demyelination increases radial diffusivity in corpus callosum of mouse brain. Neuroimage. 2005;26(1):132–140. doi: 10.1016/j.neuroimage.2005.01.028. [DOI] [PubMed] [Google Scholar]
- Srinivasan R. Spatial structure of the human alpha rhythm: global correlation in adults and local correlation in children. Clinical Neurophysiology. 1999;1999(110):1351–1362. doi: 10.1016/s1388-2457(99)00080-2. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Adalsteinsson E, Hedehus M, Ju C, Moseley M, Lim KO, et al. Equivalent disruption of regional white matter microstructure in ageing healthy men and women. Neuroreport. 2001;12(1):99–104. doi: 10.1097/00001756-200101220-00027. [DOI] [PubMed] [Google Scholar]
- Sun SW, Liang HF, Trinkaus K, Cross AH, Armstrong RC, Song SK. Noninvasive detection of cuprizone induced axonal damage and demyelination in the mouse corpus callosum. Magn Reson Med. 2006;55(2):302–308. doi: 10.1002/mrm.20774. [DOI] [PubMed] [Google Scholar]
- Takao M, Koto A, Tanahashi N, Fukuuchi Y, Takagi M, Morinaga S. Pathologic findings of silent hyperintense white matter lesions on MRI. Journal of the Neurological Sciences. 1999;167:127–131. doi: 10.1016/s0022-510x(99)00158-6. [DOI] [PubMed] [Google Scholar]
- Tang Y, Nyengaard JR, Pakkenberg B, Gundersen HJ. Age-induced white matter changes in the human brain: a stereological investigation. Neurobiol Aging. 1997;18:609–615. doi: 10.1016/s0197-4580(97)00155-3. [DOI] [PubMed] [Google Scholar]
- Tombaugh TN. Trail Making Test A and B: normative data stratified by age and education. Arch Clin Neuropsychol. 2004;19(2):203–214. doi: 10.1016/S0887-6177(03)00039-8. [DOI] [PubMed] [Google Scholar]
- Tuch DS, Salat DH, Wisco JJ, Zaleta AK, Hevelone ND, Rosas HD. Choice reaction time performance correlates with diffusion anisotropy in white matter pathways supporting visuospatial attention. Proc Natl Acad Sci U S A. 2005;102(34):12212–12217. doi: 10.1073/pnas.0407259102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turken A, Whitfield-Gabrieli S, Bammer R, Baldo JV, Dronkers NF, Gabrieli JD. Cognitive processing speed and the structure of white matter pathways: convergent evidence from normal variation and lesion studies. Neuroimage. 2008;42(2):1032–1044. doi: 10.1016/j.neuroimage.2008.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermathen P, Robert-Tissot L, Pietz J, Lutz T, Boesch C, Kreis R. Characterization of white matter alterations in phenylketonuria by magnetic resonance relaxometry and diffusion tensor imaging. Magn Reson Med. 2007;58:1145–1156. doi: 10.1002/mrm.21422. [DOI] [PubMed] [Google Scholar]
- Vernooij MW, Ikram MA, Vrooman HA, Wielopolski PA, Krestin GP, Hofman A, et al. White matter microstructural integrity and cognitive function in a general elderly population. Arch Gen Psychiatry. 2009;66(5):545–553. doi: 10.1001/archgenpsychiatry.2009.5. [DOI] [PubMed] [Google Scholar]
- Walhovd KB, Fjell AM, Reinvang I, Lundervold A, Dale AM, Eilertsen DE, et al. Effects of age on volumes of cortex, white matter and subcortical structures. Neurobiol Aging. 2005;26(9):1261–1270. doi: 10.1016/j.neurobiolaging.2005.05.020. [DOI] [PubMed] [Google Scholar]
- Waxman SG. Conduction in myelinated, unmyelinated, and demyelinated fibers. Arch Neurol. 1977;34(10):585–589. doi: 10.1001/archneur.1977.00500220019003. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale - Revised. San Antonio: Psychological Corporation; 1981. [Google Scholar]
- Weiss KL, Schroeder CE, Kastin SJ, Gibson JP, Yarrington JT, Heydorn WE, et al. MRI monitoring of vigabatrin-induced intramyelinic edema in dogs. Neurology. 1994;44(10):1944–1949. doi: 10.1212/wnl.44.10.1944. [DOI] [PubMed] [Google Scholar]
- Wilkinson R, Allison S. Age and simple reaction time: decade difference for 5,325 subjects. J Geront Psych Sci. 1989;44:29–35. doi: 10.1093/geronj/44.2.p29. [DOI] [PubMed] [Google Scholar]
- Yakovlev PI, Lecours AR. Regional Development of the Brain in Early Life. Boston: Blackwell Scientific Publications; 1967. [Google Scholar]
- Zhan L, Leow AD, Zhu S, Baryshev M, Toga AW, McMahon KL, et al. A novel measure of fractional anisotropy based on the tensor distribution function. Med Image Comput Comput Assist Interv. 2009;12(Pt 1):845–852. doi: 10.1007/978-3-642-04268-3_104. [DOI] [PubMed] [Google Scholar]