Abstract
Iron acquisition is essential for Mycobacterium tuberculosis (Mtb) virulence. Understanding the molecular mechanisms used by Mtb to scavenge iron during infection might reveal new targets for antimicrobial development. Rv2895c, a homolog of ViuB from Vibrio cholerae has been postulated to be involved in iron-siderophore uptake and utilization in Mtb. This study examines the requirement of Rv2895c for adaptation of Mtb to iron limitation. We show that Rv2895c is dispensable for normal replication of Mtb in iron deficient conditions and in human macrophages. Thus, contrary to the predictions of sequence analysis and in vitro studies the genetic evidence indicates that in normal conditions Rv2895c is not required for iron acquisition in Mtb.
Keywords: siderophore, iron transport, mycobactin, FAD
Iron is an essential nutrient for most living organisms including Mycobacterium tuberculosis (Mtb). Due to the poor solubility of ferric iron (Fe+3) in the presence of oxygen and at neutral pH, free iron is not found in the mammalian host, but sequestered in complexes with proteins such as transferrin, lactoferrin and ferritin.1 Acquiring iron during infection poses a challenge for most pathogens. In bacteria, a common mechanism to acquire iron is the synthesis and secretion of high affinity iron chelators (siderophores) that can solubilize iron and efficiently compete with iron-binding proteins of the host. Fe+3-siderophore complexes are transported into the cell by specific importers. In the cytosol, Fe+3 is dissociated from the siderophore via cleavage of the siderophore or by the action of a ferric reductase. Reduction of Fe+3 results in a weaker binding of Fe+2 to the siderophore, allowing release of iron.2
Iron uptake systems are attractive targets for the development of antimicrobial agents that interfere with iron acquisition. Mtb synthesizes siderophores named mycobactins. One form of mycobactin is very hydrophobic and remains cell associated while carboxymycobactin (also known as exomycobactin) is more hydrophilic, and is secreted.3 The operon encoding the ABC permeases IrtA and IrtB is required for uptake of iron from Fe+3-carboxymycobactin and growth of Mtb in iron deficient conditions.4 The cytosolic, amino terminal domain of IrtA (IrtA-NTD) binds one molecule of flavin adenine dinucleotide (FAD) and this property is essential for iron acquisition through IrtAB.5 Since most bacterial ferric reductases are flavin reductases6, IrtA-NTD might be a ferric-reductase of Fe+3-carboxymycobactin imported by IrtAB. IrtA-NTD is highly similar to Vibrio cholerae’s ViuB, a cytosolic protein required for the utilization of ferric-vibriobactin 5,7 ViuB homologs are present in several bacteria and are annotated as siderophore interacting proteins. Structural studies of the ViuB homologs of Shewanella putrefaciens and E. coli (YqjH) support a role of these proteins as NAD(P)H:flavin oxidoreductases 8.
In addition to the IrtA-NTD, the cytosolic protein encoded by Rv2895c shows 31% similarity to ViuB, and it has been annotated as the Mtb ViuB possibly functioning as a siderophore utilization protein (genolist.pasteur.fr/TubercuList). Rv2895c is induced in Mtb growing in low iron9 and in BCG replicating in mouse macrophages.10 Based on physical interactions detected in-vitro between recombinant Rv2895c and IrtB as well as Rv2895c and Fe+3 -carboxymycobactin, it was suggested that Rv2895c acted as siderophore binding protein for iron transport by IrtB.9,11 Given the potential of iron transporters as drug targets, identification of the proteins involved in iron acquisition in Mtb is important. In this study we sought a genetic proof of the role of Rv2895c in iron acquisition. An Rv2895c mutant in Mtb H37Rv was generated and the effect of the deletion on the adaptation of Mtb to low iron was evaluated. A 0.7 kb region encoding 249 amino acids of the 283 constituting Rv2895c was deleted and replaced with a hygromycin resistant cassette by specialized transduction and homologous recombination.12 The mutant (ST212) was confirmed using Southern blot (figure 1) and PCR analysis (data not shown). Growth of the Rv2895c mutant (ST212) strain was equivalent to the wild type in standard 7H9, which is an iron rich medium containing 100–165uM iron depending upon the source of ferric ammonium citrate. We next examined the effect of Rv2895c deletion on the ability of Mtb to replicate under iron deficient conditions. To assess replication under iron deficient conditions the strains were grown in a defined low iron (~2uM FeCl3) medium (MM).4 A mutant of the iron transporter IrtAB (ST73)4 was used as control. Growth was assessed as a function of increase in optical density at 540nm. ST212 grew as well as the wild type strain under iron deficiency (figure 2A), whereas the same conditions limited the growth of the iron transport mutant ST73 (figure 2B). Further reduction of iron in the cells was achieved by addition of the permeable ferrous iron chelator 2,2′-dipyridyl (DPI) to the growth medium. DPI drastically reduced growth of ST73 (figure 2D) but did not affect growth of ST212 (figure 2C). These results show that disruption of Rv2895c does not alter the ability of Mtb to replicate under iron deficient conditions and indicate that Rv2895c is dispensable for iron acquisition in Mtb. To examine whether deletion of Rv2895c could have a minor effect in iron acquisition, not detected in the growth assay, we determined the MIC of streptonigrin for the wild type and the mutant strains. Streptonigrin is used as an indirect indicator of intracellular iron levels since this antibiotic requires iron for its bactericidal activity.13 Indeed, iron deficient mutants exhibit increased resistance to streptonigrin than their parental strains.5 This experiment was conducted in MM and the MIC was determined using the microplate Alamar Blue Assay.14 MIC was defined as the concentration that inhibit at least 90% growth. Wild type and ST212 strains exhibit the same MIC for streptonigrin (2.5 ug/ml) indicating that there is no detectable difference in the intracellular levels of iron in the wild type and the Rv2895c mutant.
Figure 1.
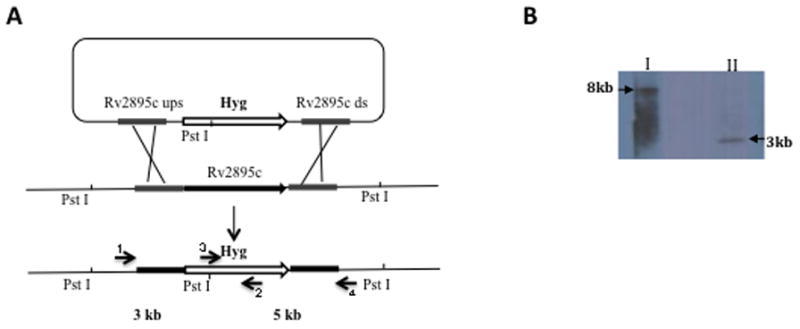
Strategy for generation of a knock out mutant of Rv2895c in H37Rv. The Rv2895c gene was replaced with a hygromycin (Hyg) resistance cassette by phage transduction and homologous recombination. A) Scheme of the genome region containing Rv2895c and the substrate used for homologous recombination. Position of the PstI restriction sites used for chromosomal DNA digestion and the primers used for PCR amplification. B) Southern blot to confirm the ST212 mutant. The genomic DNA of H37Rv and ST212 was completely digested with PstI enzyme and the blot was probed with a fragment containing the 500 bp upstream Rv2895c. The PstI restriction sites in the vicinity of Rv2895c in wild type H37Rv are 8 kb apart (I) whereas restriction of ST212 DNA generates a 3 kb band (II), since the Hyg cassette has an internal PstI site. Gene replacement was also confirmed by PCR and sequencing. Primers 1 and 4 were designed in the region upstream and downstream of Rv2895c. Primers 2 and 3 are internal primers of the Hyg cassette. PCR was done using primers 1 and 2 and primers 3 and 4 with DNA from both wild type and mutant. No product was obtained using the wild type DNA whereas products of 2kb and 1.4kb were obtained using DNA isolated from the mutant as template. Sequencing of the PCR products confirmed the homologous recombination and gene replacement.
Figure 2.
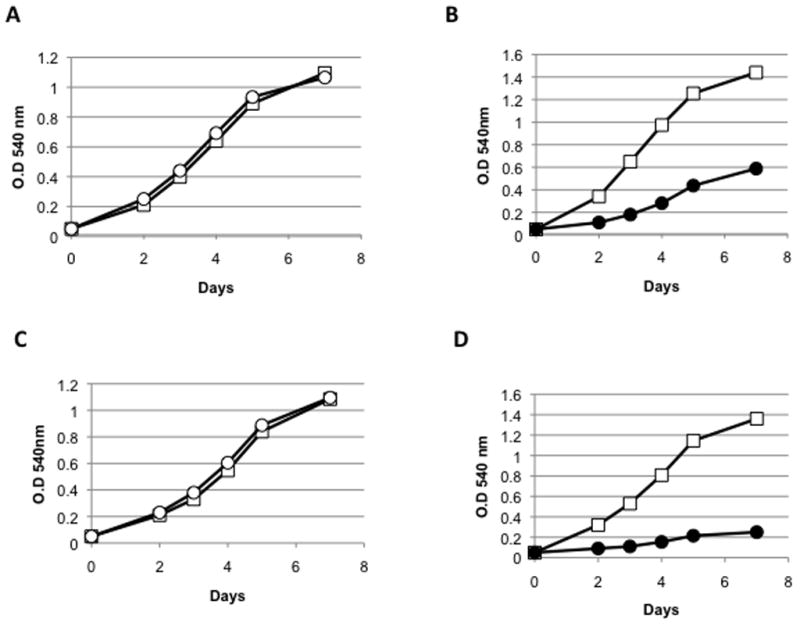
Comparison of the growth of M. tuberculosis strains ST212 (○) and ST73 (●) with wild type H37Rv (□). (A and B). Growth in iron deficient medium (MM) (0.5 % w/v asparagine, 0.5% w/v KH2P04, 2% glycerol, 0.05% Tween-80 and 10% albumin-dextrose-NaCl complex (ADN), 0.5 mg ZnCl2/L, 0.1 mg/L MnS04, and 40 mg/L MgS04). Strains were passed two times in MM to generate iron starvation and then the growth was monitored by measuring change in optical density. Strains were grown at 37° C with agitation. (C and D) Growth in iron-deficient medium containing 75μM DPI. One representative experiment is shown. The experiments were repeated three times.
Macrophages provide an iron-limiting environment for Mtb and siderophore mediated iron acquisition is required for normal replication of Mtb in these cells.13,4 To assess the effect of Rv2895c deletion on Mtb’s replication in human macrophages, THP-1 cells were differentiated into macrophages and infected with ST212 and H37Rv as described before.4 As shown in figure 3, ST212 replicated similarly to the wild type in THP-1 cells indicating that Rv2895c is not necessary to overcome iron deficiency in macrophages. Could IrtA-NTD and Rv2895c have a redundant function? This is unlikely considering that even a single amino amino-acid substitution in the FAD binding domain of IrtA-NTD practically abolishes growth in low iron despite the presence of Rv2895c.5 Rv2895c may not be able to function as IrtA-NTD because of its localization, inability to bind FAD or other reasons. To examine the ability of Rv2895c to bind FAD, the protein was over expressed and purified in E. coli. The expressed protein was mostly insoluble. The protein was purified from inclusion bodies under denaturing conditions and then solubilised by step-wise dialysis and removal of the denaturant. FAD was included in the final step of dialysis and during an additional overnight incubation at a protein:FAD ratio of 1:3. Upon removal of unbound FAD the UV/visible absorbance spectrum of the solubilised protein indicated no binding of FAD (data not shown). It is possible that the protein did not refold properly into the native conformation or alternatively it does not bind FAD. Since one of the critical FAD binding residues Tyr 72 in IrtA-NTD, which is conserved in ViuB5, has been replaced by a Met in Rv2895c, it is possible that this change resulted in loss of FAD binding activity.
Figure 3.

Replication of M. tuberculosis strains in THP-1 cells. THP-1 cells were induced to differentiate into macrophages5 and infected with H37Rv and ST212. The cells were lysed to release mycobacteria on select days and were plated onto 7H10 to determine CFU. The reported values represent the averages and standard deviations of three parallel independent infections. Strains: H37Rv (◆); ST212 (▲).
Taken together, the results of this study indicate that despite the similarity to ViuB and IrtA-NTD, under normal conditions, Rv2895c is dispensable for iron acquisition in Mtb. Regarding the biochemical interaction observed in a previous study between Rv2895c and IrtB9 it is not unusual that physical interactions observed with cell lysates do not actually represent functional interactions in vivo.
Acknowledgments
We thank Dr. Issar Smith and the Rodriguez lab for helpful discussions. This study was supported by NIH grant AI044856 (GMR).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Weinberg ED. Iron loading and disease surveillance. Emerg Infect Dis. 1999;5:346–52. doi: 10.3201/eid0503.990305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miethke M, Marahiel MA. Siderophore-based iron acquisition and pathogen control. Microbiol Mol Biol Rev. 2007;71(3):413–51. doi: 10.1128/MMBR.00012-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Snow GA. Mycobactins: iron chelating growth factors from mycobacteria. Bacteriol Rev. 1970;34:99–125. doi: 10.1128/br.34.2.99-125.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rodriguez GM, Smith I. Identification of an ABC Transporter Required for Iron Acquisition and Virulence in Mycobacterium tuberculosis. J Bacteriol. 2006;188(2):424–30. doi: 10.1128/JB.188.2.424-430.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ryndak MB, Wang S, Smith I, Rodriguez GM. The Mycobacterium tuberculosis high-affinity iron importer, IrtA, contains an FAD-binding domain. J Bacteriol. 192(3):861–9. doi: 10.1128/JB.00223-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schroder I, Johnson E, de Vries S. Microbial ferric iron reductases. FEMS Microbiol Rev. 2003;27(2–3):427–47. doi: 10.1016/S0168-6445(03)00043-3. [DOI] [PubMed] [Google Scholar]
- 7.Butterton JR, Calderwood SB. Identification, cloning, and sequencing of a gene required for ferric vibriobactin utilization by Vibrio cholerae. J Bacteriol. 1994;176(18):5631–8. doi: 10.1128/jb.176.18.5631-5638.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bamford VA, Armour M, Mitchell SA, Cartron M, Andrews SC, Watson KA. Preliminary X-ray diffraction analysis of YqjH from Eschrichia coli: a putative cytoplasmic ferri-siderophore reductase. Crystallization communications. 2008;64:792–796. doi: 10.1107/S174430910802352X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farhana A, Kumar S, Rathore SS, Ghosh PC, Ehtesham NZ, Tyagi AK, Hasnain SE. Mechanistic insights into a novel exporter-importer system of Mycobacterium tuberculosis unravel its role in trafficking of iron. PLoS ONE. 2008;3(5):e2087. doi: 10.1371/journal.pone.0002087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Srivastava V, Rouanet C, Srivastava R, Ramalingam B, Locht C, BSS Macrophage-specific Mycobacterium tuberculosis genes:identification by green fluorescence protein and kanamycin resistance selection. Microbiology. 2007;153:659–666. doi: 10.1099/mic.0.2006/000547-0. [DOI] [PubMed] [Google Scholar]
- 11.Banerjee S, Farhana A, Ehtesham NZ, Hasnain SE. Iron acquisition, assimilation and regulation in mycobacteria. Infect Genet Evol. 2010;11(5):825–38. doi: 10.1016/j.meegid.2011.02.016. [DOI] [PubMed] [Google Scholar]
- 12.Bardarov S, Bardarov S, Jr, Pavelka MS, Jr, Sambandamurthy V, Larsen M, Tufariello J, Chan J, Hatfull G, Jacobs WR., Jr Specialized transduction: an efficient method for generating marked and unmarked targeted gene disruptions in Mycobacterium tuberculosis. M. bovis BCG and M. smegmatis. Microbiology. 2002;148(Pt 10):3007–17. doi: 10.1099/00221287-148-10-3007. [DOI] [PubMed] [Google Scholar]
- 13.Yeowell HN, White JR. Iron requirement in the bactericidal mechanism of streptonigrin. Antimicrob Agents Chemother. 1982;22(6):961–8. doi: 10.1128/aac.22.6.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Franzblau SG, Witzig RS, McLaughlin JC, Torres P, Madico G, Hernandez A, Degnan MT, Cook MB, Quenzer VK, Ferguson RM, Gilman RH. Rapid, low-technology MIC determination with clinical Mycobacterium tuberculosis isolates by using the microplate Alamar Blue assay. J Clin Microbiol. 1998;36(2):362–6. doi: 10.1128/jcm.36.2.362-366.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]


