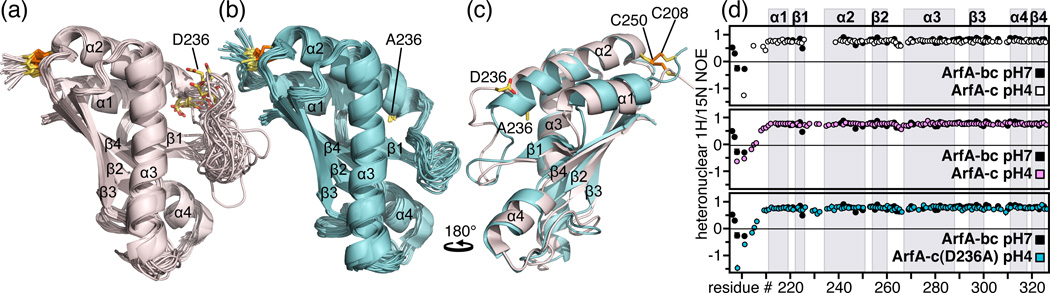
Figure 1. Structures and backbone dynamics of wild-type ArfA-c and ArfA-c(D236A).
(a, b) Backbone representations of the 20 lowest energy structures of (a) wild-type ArfA-c and (b) ArfA-c(D236A). (c) Superimposed lowest energy structures of ArfA-c (pink) and ArfA-c(D236A) (cyan). The structures were determined at pH7. The average pairwise RMSDs for the structured core of the protein (residues 207–326) are reported in Table S1. (d) Heteronuclear 1H/15N NOEs reflecting backbone dynamics of: ArfA-bc at pH7 (black), ArfA-c at pH7 (white), ArfA-c at pH4 (pink), and ArfA-c(D236A) at pH7 (cyan). For wild-type ArfA-c, peaks from residues in the β1-α2 loop could not be observed at pH7. Residues in this loop gave weak, broad peaks at pH4, but gave peaks of normal intensity in the D236A mutant.