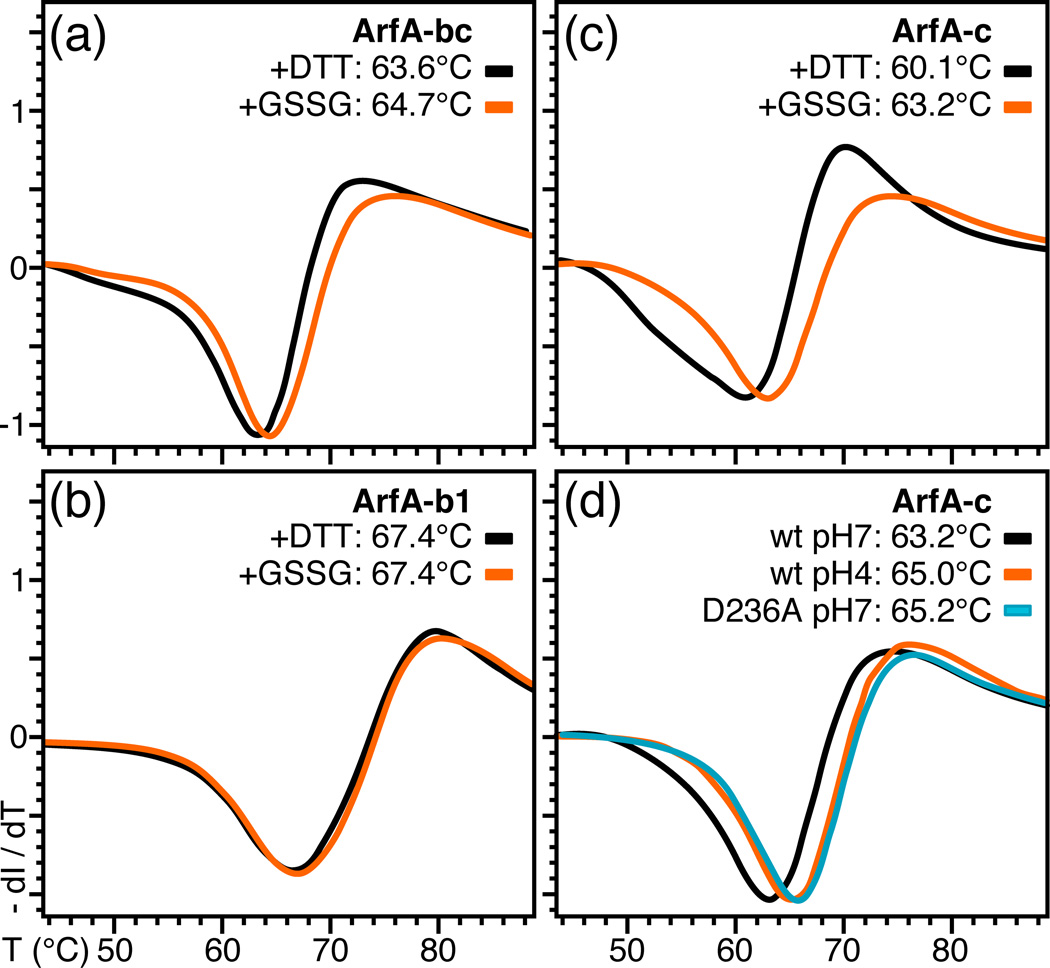
Figure 2. Differential scanning fluorimetry traces showing the effect of the disulfide bond, pH, and D236A mutation on the thermal stability of ArfA.
(a–c) Protein was treated with DTT or GSSG to break or form the C208–C250 disulfide bond. ArfA-b1 encompasses residues 73 to 220. (d) Traces were obtained for wild-type ArfA-c at pH7 (black) or pH4 (orange) and for ArfA-c(D236A) at pH7 (cyan).