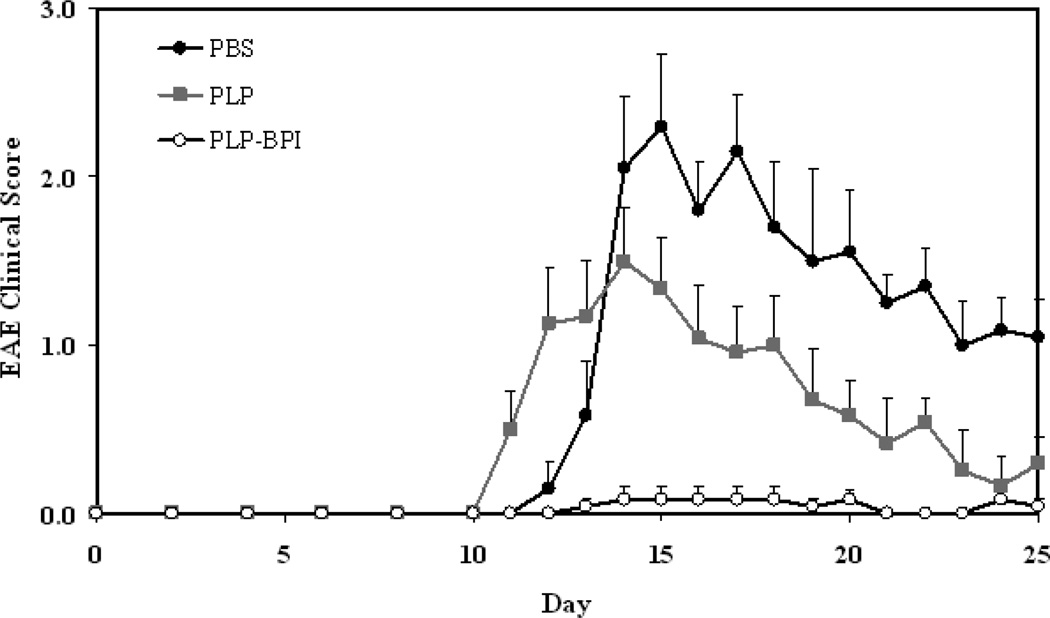
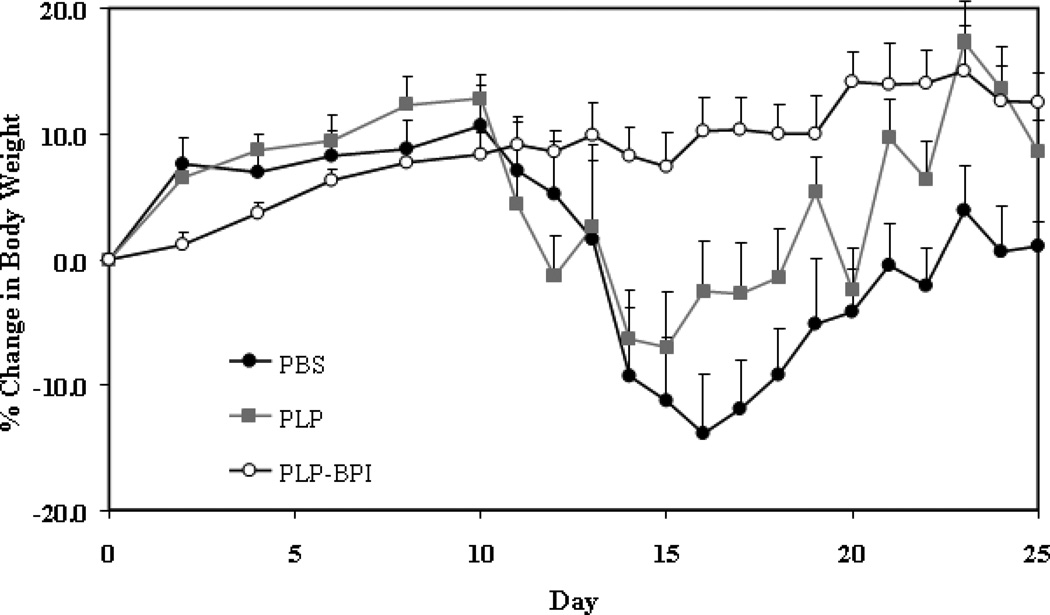
Figure 2.
In vivo efficacies of PLP-BPI and PLP in suppressing EAE in the mouse model upon vaccination with peptides and immunization with PLP/CFA on day 0. PBS-treated mice received subcutaneous injections of 100 µl PBS on days −11, −8, and −5. PLP-BPI- and PLP-treated mice received 100 nmol/100 µl PBS on days −11, −8, and −5. The efficacy of the peptide was determined by (A) clinical disease score of EAE and (B) percent change in body weight. Results are expressed as the mean ± SEM (n = 12). EAE scores from all PLP-BPI treated mice were significantly lower than those of PBS- and PLP-treated mice (p < 0.0001). Loss of body weight was also significantly lower in PLP-BPI-treated mice compared to those treated with PBS (p < 0.0001) and PLP (p < 0.001). For statistical analysis, data points from days 10 to 25 were used.