Figure 1.
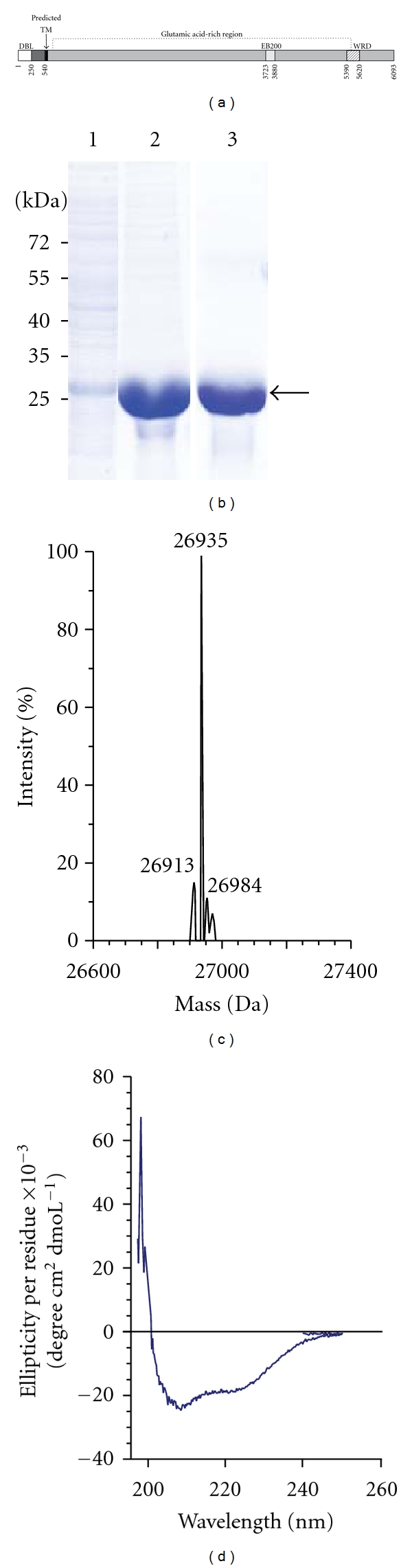
Purification and characterization of recombinant Pf332-DBL. (a) Schematic representation of the full-length Pf332 protein. Residues 1–570 are encoded by the first exon, which contains the Duffy-binding-like (DBL) domain (white) and the predicted transmembrane domain (black). Exon 2 encodes the extensive glutamic acid-rich repeat region (grey), which contains the repetitive EB200 region (light gray) and the tryptophan-rich WRD region (striped). (b) Coomassie-stained SDS polyacrylamide gel after each purification step of Pf332-DBL. Lane 1: E. coli soluble lysate, lane 2: after affinity purification on a Ni-column, and lane 3: after size exclusion chromatography. Bands corresponding to recombinant Pf332-DBL protein are indicated. (c) Mass spectrum profile of Pf332-DBL after size exclusion chromatography to verify purity. One major ion peak at m/z 26935 can be visualized. (d) Circular dichroism spectrum of Pf332-DBL in the far UV region, demonstrating that the protein is folded and consisting predominantly of α-helices, as indicated by a positive band at 195 nm and two negative bands at 208 and 220 nm.
