Abstract
The effects of acidic polysaccharides purified from Gastrodia rhizome on blood pressure and serum lipid levels in spontaneously hypertensive rats (SHR) fed a high-fat diet were investigated. Acidic polysaccharides were purified from crude polysaccharides by DEAE-Sepharose CL-6B. Thirty-six male SHR were randomly divided into three groups: Gastrodia rhizome crude polysaccharide (A), acidic polysaccharide (B) groups, and a control group (C). A 5-week oral administration of all treatment groups was performed daily in 3- to 8-week-old SHRs with a dose of 6 mg/kg of body weight/day. After 5 weeks of treatment, total cholesterol in the acidic polysaccharide group, at 69.7 ± 10.6 mg/dL, was lower than in the crude polysaccharide group (75.0 ± 6.0 mg/dL) and the control group (89.2 ± 7.4 mg/dL). In addition, triglyceride and low-density lipoprotein cholesterol levels in the acidic polysaccharide group were lower than in the crude polysaccharide and control groups. The atherogenic index of the acidic polysaccharide group was 46.3% lower than in the control group. Initial blood pressure after the initial three weeks on the high-fat diet averaged 195.9 ± 3.3 mmHg among all rats. Compared with the initial blood pressure, the final blood pressure in the control group was increased by 22.8 mmHg, whereas it decreased in the acidic polysaccharide group by 14.9 mmHg. These results indicate that acidic polysaccharides from Gastrodia rhizome reduce hypertension and improve serum lipid levels.
Keywords: Gastrodia rhizome, acidic polysaccharide, serum lipid level, blood pressure
1. Introduction
Hypertension, also known as high blood pressure, is a major public health problem due to its high prevalence and complications [1]. It is defined as a systolic blood pressure of 140 mmHg or higher, and a diastolic blood pressure of 90 mmHg or higher [2]. One of the factors contributing to the development of hypertension is life style and eating habits, such as high consumption of salt, alcohol, cholesterol and fat [3]. Over consumption of salt, alcohol, cholesterol and fat have resulted in alarming increases in the incidences of hypertension, insulin resistance, and obesity [4]. On the other hand, current research suggests that fruits and vegetables contain polysaccharides and phytochemicals that substantially lower blood pressure and blood lipid levels [5,6]. In addition, several reports have been published on anti-hypertensive effect of natural polysaccharide and presented the following: the polysaccharides fraction from American ginseng berry extract has a significant anti-hyperglycemic activity [7]. Treatment with polysaccharide fraction of mushroom species such as Pleurotus nebrodensis in SHR showed antihypertensive effect via the inhibition of angiotensin conversion [8]. We previously reported [9] that the anti-hypertensive action of macromolecules such as crude polysaccharides from Gastrodia rhizome in SHR is significantly stronger than that of the protein fraction and low-molecular-weight fraction (phenolic compound).
Gastrodia rhizome, a polysaccharide-enriched dry tuber of Gastrodia elata Blume (Orchidaceae), has been used in traditional medicine in Korea, Japan and China as an anti-convulsant, an analgesic, and a sedative against general paralysis, epilepsy, vertigo, and tetanus [10]. The major physiological substances of Gastrodia elata Blume are gastrodin, parishin, and vanillyl alcohol, glycoprotein, in addition to polysaccharides, including α-d-glucan [11–13]. Among the components of Gastrodia elata Blume, glycoproteins inhibit platelet aggregation and exhibit antithrombosis activity [11]. Tong and others [14] reported that sulfated α-d-glucan from Gastrodia elata Blume exerts a potent inhibitory effect on dengue virus serotype 2. Although Gastrodia rhizome has various biological actions [15–18], the antihypertensive effect of acidic polysaccharides purified from Gastrodia rhizome in SHR fed a high-fat diet has not yet been studied.
This study therefore investigated the antihypertensive potential of acidic polysaccharides purified from Gastrodia rhizome and also evaluated the concentrations of serum lipids, including total cholesterol (TC), triglyceride (TG), high-density lipoprotein (HDL) cholesterol, and low-density lipoprotein (LDL) cholesterol in SHR fed a high-fat diet.
2. Results and Discussion
2.1. Characterization of Acidic Polysaccharides from Gastrodia Rhizome
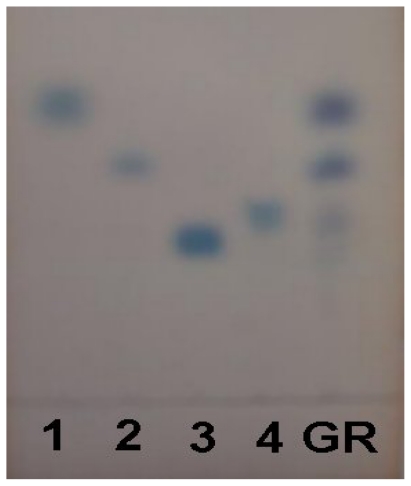
In our previous study [9], a hot-water extract of Gastrodia rhizome was fractionated to obtain a crude polysaccharide (GR-0). The crude polysaccharide exhibited the most potent antihypertensive action compared with the protein fraction and low-molecular-weight fraction [9]. It was further fractionated by ion-exchange chromatography on DEAE-Sepharose CL-6B. As shown in Table 1, crude polysaccharide (GR-0) contained 1.19 ± 0.11% of total protein, 86.25 ± 1.54% of total sugar, and 12.56 ± 1.15% of acidic polysaccharides, whereas the acidic polysaccharide fractions of DEAE-Sepharose CL-6B contained 0.32 ± 0.02% of total protein, 82.40 ± 1.16% of total sugar, and 17.28 ± 0.58% of acidic polysaccharides, respectively. The fraction of acidic polysaccharides from Gastrodia rhizome might consist of simple monosaccharide of six carbon atoms. According to the retention factor, the fraction of acidic polysaccharides included xylose, glucose, galacturonic acid, and glucuronic acid (Figure 1).
Table 1.
Chemical compositions and yields of the crude polysaccharides and the acidic polysaccharide fractions purified from Gastrodia rhizome.
| Yield (g) | Total protein (%) | Total sugar (%) | Acidic polysaccharides (%) | |
|---|---|---|---|---|
| Crude polysaccharides fraction | 2.47 | 1.19 ± 0.11 | 86.25 ± 1.54 | 12.56 ± 1.15 |
| Acidic polysaccharide fraction | 0.61 | 0.32 ± 0.02 | 82.40 ± 1.16 | 17.28 ± 0.58 |
Figure 1.
Thin-layer chromatography (TLC) patterns of acid hydrolyzate of acidic polysaccharides from Gastrodia rhizome. 1: xylose; 2: glucose; 3: galacturonic acid; 4: glucuronic acid; GR: acid hydrolyzate of acidic polysaccharides from Gastrodia rhizome.
2.2. Antihypertensive Effect of Acidic Polysaccharides from Gastrodia Rhizome
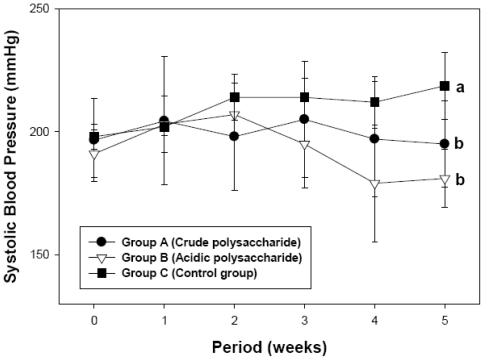
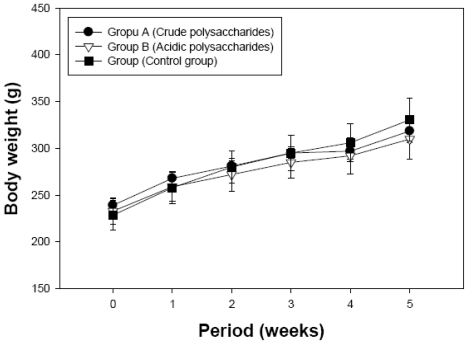
To determine the effect of acidic polysaccharides on hypertension in SHR fed a high-fat diet, systolic blood pressure was evaluated. Reference blood pressure (RBP), after consuming the high-fat diet for an initial 3 weeks, averaged 195.9 ± 3.3 mmHg. Figure 2 shows that the blood pressure of Group C (control) increased from 195.9 ± 3.3 mmHg to 218.7 ± 13.6 mmHg in 5 week of treatment period. However, the blood pressure of Group A and Group B was 195.0 ± 17.6 and 181.0 ± 11.87 mmHg, respectively, in the same period. The crude and acidic polysaccharide groups showed significantly lower blood pressure than the control group (p < 0.05). Compared with the initial blood pressure (195.9 mm Hg), the final blood pressure of the control group increased by 22.8 mmHg, but that of the acidic polysaccharide group decreased by 14.9 mmHg. As shown in Figure 3, no significant difference (p > 0.05) was observed in body weight between both polysaccharide groups and the control group during the 5-week treatment period. Our results are consistent with those of Zhu and others [19], who reported that the d-polymannuronic sulfate markedly reduces systolic blood pressure (SBP) and diastolic blood pressure (DBP) dose-dependently and decreases heart rate (HR) with a reduction in arterial blood pressure. Ding and others [11] showed that polysaccharide 2-1 faction from Gastrodia elata has remarkable anticoagulation and anti-thrombosis effects in mice and in vitro models. The antihypertensive activity of Group A and B may depend on the amount of acidic polysaccharides in the fractions (Table 1 and Figure 2).
Figure 2.
Effects of crude and acidic polysaccharides of Gastrodia rhizome on blood pressure after 5 weeks of treatment. * p < 0.05 indicates statistically significant different to only control group (Group C); also data are presented as the mean ± standard deviation of twelve experiments in each group.
Figure 3.
Effects of crude and acidic polysaccharides of Gastrodia rhizome on body weight after 5 weeks of treatment. No significant difference was observed between Groups A and B and control (Group C) at p > 0.05. The body weight was recorded weekly during the 5 weeks experimental period. Values are expressed as the mean ± standarddeviation.
2.3. Effects of Acidic Polysaccharides from Gastrodia Rhizome on Serum Lipid Levels
As shown in Table 2, serum TC was higher in the control group, at 89.2 mg/dL, than in both Gastrodia rhizome groups (group A, 75.0 mg/dL and group B, 69.7 mg/dL). Similarly, the administration of crude and acidic polysaccharides significantly decreased TG by 20.2 and 21.4% and LDL-cholesterol by 14.7 and 22.4%, respectively, compared to the control group. However, HDL-cholesterol in SHR treated with crude and acidic polysaccharides significantly increased by 6.7 and 14.8%, respectively. At the same time, AI in the acidic polysaccharide group was 46.3% lower than in the control group.
Table 2.
Effects of crude and acidic polysaccharides from Gastrodia rhizome on serum lipid levels and AI in spontaneously hypertensive rats (SHR) fed a high-fat diet.
| Group (n = 12) | Lipids (mg/dL) and AI | ||||
|---|---|---|---|---|---|
| TC 1 | TG 2 | HDL 3 | LDL 4 | AI 5 | |
| A | 75.0 6 ± 6.0 b | 133.0 ± 9.4 b | 28.8 ± 1.6 b | 24.4 ± 1.8 b | 1.61 ± 0.31 b7 |
| B | 69.7 ± 10.6 b | 131.0 ± 8.7 b | 31.0 ± 2.0 a | 22.2 ± 3.6 b | 1.24 ± 0.22 c |
| C | 89.2 ± 7.4 a | 166.7 ± 16.3 a | 27.0 ± 0.9 b | 28.6 ± 3.8 a | 2.31 ± 0.27 a |
TC: total cholesterol;
TG: triglyceride;
HDL: high-density lipoprotein cholesterol;
LDL: low-density lipoprotein cholesterol;
AI: atherogenic index ((TC-HDL)/HDL);
Values are mean ± SD (n = 12);
Means in the same column with different superscript letters are significantly different (p < 0.05).
Our results reveal that both crude (Group A) and acidic polysaccharides (Group B) are able to reduce blood pressure (Figure 2), TC, TG, and LDL (Table 2) in SHR fed a high-fat diet. After 5 weeks of treatment, blood pressure (181.0 ± 11.8 mmHg) in SHR treated with acidic polysaccharides was lower than in the crude polysaccharide (195.0 ± 17.6 mmHg) and control groups (218.7 ± 13.6 mmHg). Compared with the control group, the administration of the acidic polysaccharides significantly decreased blood pressure by 17.2% (Figure 2). Furthermore, Group B decreased TC by 21.9%, TG by 21.4%, LDL-cholesterol by 22.4%, and AI by 46.3% (Table 2). In addition, serum HDL-cholesterol was markedly higher in the Group B, at 31.0 ± 2.0 mg/dL, than in the control (Group C, p < 0.05). During the experimental period, no significant differences were observed in body weight (Figure 3), food intake, or water intake (data not shown) between either polysaccharide groups or the control group.
Hypertension is one of the risk factors for stroke, which is associated with age, gender, elevated cholesterol, smoking, alcohol, excessive weight, and family history [20]. Recently, antihypertensive agents were suggested by various mechanisms. Inhibition of angiotensin I converting enzyme (ACE), one of antihypertensive mechanism, has been used for evaluating antihypertensive activity of natural products and functional food [21]. ACE removes a dipeptide from the C terminus of angiotensin I to form angiotensin II, a highly reactive hypertensive compound [20]. A few reports have investigated that polysaccharide from food ingredients present hypertensive activity via inhibition of ACE activity. Miyazawa and others [8] showed that polysaccharide fraction of mushroom species such as Pleurotus nebrodensis in SHR reduced blood pressure via the inhibition of angiotensin conversion. In addition, pectin hydroxamic acid shows the dose-dependent inhibitory activity of ACE [20]. Therefore, our results also suggest that the administration of the acidic polysaccharide distinctly retarded the development of hypertension in SHR via inhibition of ACE activity. However, these enzyme assays need to be examined in the future to better understand the mechanism of acidic polysaccharides from Gastrodia rhizome on antihypertensive activity.
3. Experimental Section
3.1. Materials
The Gastrodia rhizome was purchased from the Herbal Medicine Co-operative of Muju, Chonbuk, South Korea. Cleaned Gastrodia rhizome was peeled, thinly sliced, and dried at 40 ± 5 °C for 6 h. The dried Gastrodia rhizome was ground to 20–30 mesh using a grinder (IKA M 20, IKA, Staufen, Germany).
3.2. Preparation of Crude and Acidic Polysaccharides
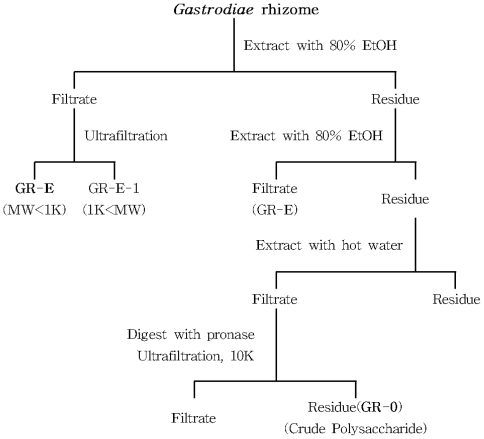
The Gastrodia rhizome powder was refluxed with 10 volumes (v/w) of 80% ethanol at 60 °C for 3 h, and the extraction was repeated two times. The extracts were filtered through Whatman filter paper (No. 2). The precipitates were extracted with 2 L boiling distilled H2O for 3 h. The aqueous extract was centrifuged at 2000× g for 15 min and filtered through Whatman filter paper (No. 2). Hot water extract was dissolved in 1 L of Tris-HCl buffer (pH 7.5) that contained 10 mM CaCl2, and the extract was treated with 50 mg of pronase. The reaction mixture was incubated at 37 °C for 6 days. The reaction was stopped by boiling for 5 min, followed by dialysis. The nondialyzable portion, crude polysaccharides (GR-0), was lyophilized to obtain the pronase-digested product (Figure 4). The crude polysaccharide extract was further purified using ion-exchange chromatography on a DEAE-Sepharose CL-6B (Figure 5). The crude polysaccharides were eluted by stepwise elution with 0, 0.05, 0.1, 0.2, 0.3, 0.4, and 0.5 M NaCl. It was dissolved in drinking water and used for animal test samples. Total sugar and protein contents of crude and acidic polysaccharides were determined using phenol-H2SO4 [22] and the BCA method with a BCA™ protein assay kit with BSA as the standard (Thermo Scientific, Pittsburgh, PA, USA).
Figure 4.
Isolation and preparation of crude polysaccharides from Gastrodia rhizome. Content of figure is adapted from Hong and others [9,17].
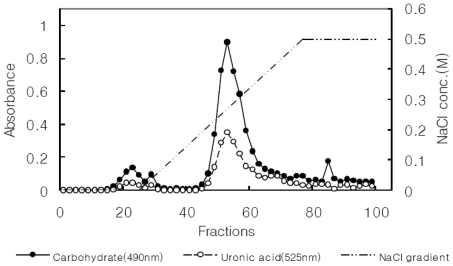
Figure 5.
Ion-exchange chromatography patterns on DEAE-Sepharose CL 6B of acidic polysaccharides from Gastrodia rhizome.
3.3. Determination of Acidic Polysaccharides Profiles
The profiles of acidic polysaccharides from Gastrodia rhizome were determined by a modified method of Thetsrimuang and others [23]. The acidic polysaccharides (3–4 mg) were hydrolyzed with 2 N HCl at 100 °C for 2–6 h. The hydrolyzed sample and standard monosaccharide were analyzed on the same thin-layer chromatography (TLC) aluminum sheet pre-coated with silica (Silica gel 60 F254, Merck, Germany). The developing agent was a mixture of acetonitrile and water (85:15, v/v). The monosaccharides were visualized on the plate after dipping into 0.5% α-naphthol in 5% H2SO4 and heating until they appeared as a dark spots. Xylose, glucose, galacturonic acid and glucuronic acid were used as standard monosaccharide.
3.4. Animal Experiments and Diet
Thirty-six male SHR (4 weeks old) were purchased from Charles River Laboratories, Inc. (Yokohama, Japan) and used after 1 week of quarantine and acclimation. Animals were kept in the animal facility of the Korea Food Research Institute in a light-controlled room (12-h light/dark cycle) at an average temperature of 24 °C and humidity of 60%. This experiment was conducted in facilities approved by the Guiding Principles for the Care and Use of Laboratory Animals of the Ethics Committee of the Korea Food Research Institute. All rats were fed an AIN-93M-based diet supplemented with high fat (10% lard, 2% corn oil, and 1% cholesterol) as the source of increased fat (Table 3). All rats were provided ad libitum access to the high-fat diet (HFD) and tap water throughout the 8-week treatment period. After three weeks on the HFD, the rats were randomly divided into three groups: Gastrodia rhizome crude polysaccharide (A) and acidic polysaccharide (B) groups and control group (C) fed only the high-fat diet. As shown in Table 4, both crude polysaccharide (6 mg/kg) and acidic polysaccharide (6 mg/kg) were administered by oral gavage to SHR. The dose of crude and acidic polysaccharides were set by considering effective dose of antihypertensive fractions of hot water and crude polysaccharide ranging from 3.6 to 14.7 mg/kg of body weight/day in SHRs [9,24]. Acidic polysaccharides were consisting of 12.56% in fraction of crude polysaccharide. Samples were administered by oral gavage in 1 mL/250 g of body weight on a daily basis 7 days per week for 5 weeks. The control animals were treated with the same volume of distilled water.
Table 3.
Composition of the experimental diet based on the AIN-93 diet with high fat.
| Ingredients | Content (%) |
|---|---|
| Casein (feed grade CP 85%) | 20.00 |
| Corn starch | 39.75 |
| Dextrinized corn starch | 13.20 |
| Sucrose | 10.00 |
| Soybean oil | 7.00 |
| Cellulose (fiber) | 5.00 |
| Mineral mixture 1 | 3.50 |
| Vitamin mixture 2 | 1.00 |
| l-Cystine | 0.30 |
| Choline bitartrate | 0.25 |
Contained per kg mixture: CaHPO4 500 g, NaCl 74 g, K3C6O7·H2O 220 g, K2SO4 52 g, MgO 24 g, 48% Mn 3.5 g, 17% Fe 6.0 g, 70% Zn 1.6 g, 53% Cu 0.3 g, KIO3 0.01 g, CrK(SO4)2·12H2O 0.55 g and sucrose.
Table 4.
Experimental design.
| Group (n = 12) | 1st phase (3 weeks) | 2nd phase (5 weeks) |
|---|---|---|
| A1 | HFD 2 | Crude polysaccharides + HFD |
| B | HFD | Acidic polysaccharides + HFD |
| C | HFD | HFD |
Groups A and B were orally administered crude and acidic polysaccharides, respectively, of Gastrodia rhizome at a concentration of 6 mg/kg, and Group C was orally administered with the same volume of distilled water, using a stainless-steel oral tube for 5 weeks.
HFD (high-fat diet): AIN diet-based commercial rat chow containing 10% lard, 2% corn oil, and 1% cholesterol (w/w).
3.5. Blood Pressure Measurements
Blood pressure was measured by a modified method of [25] in a radio frequency-shielded room. After the stabilization of animals in a 29 ± 1 °C box for 10 min, the tail systolic blood pressure was measured with a blood pressure monitor (Muromachi Kikai MK-2000, Tokyo, Japan) as the mean value of three consecutive measurements.
3.6. Blood Chemistry
At the end of the treatment, all rats fasted for 12 h were anesthetized with isoflurane and euthanized, and arteriovenous blood was collected. Blood samples were examined for serum lipid levels and atherogenic index. The concentrations of TC, TG, HDL cholesterol, and LDL cholesterol were determined using a commercial kit (Asan Phamaceutical Co., Seoul, Korea). Atherogenic index (AI) was calculated from the formula: (TC-HDL)/HDL.
3.7. Statistical Analysis
Data are presented as the means ± standard deviation. The results were statistically analyzed by one-way ANOVA and Student’s t-tests. Statistical significance was accepted at a level of p < 0.05.
4. Conclusions
The tuber of Gastrodia elata Blume, Gastrodia rhizome, has been traditionally used as a folk medicine to treat headache, hypertension, migraine, dizziness, epilepsy, infantile convulsion and tetanus in Korea and China [12,16,26]. It is also certified for use in food by the Korean Food and Drug Administration [27]. We previously reported [9] that macromolecules such as polysaccharides from Gastrodia rhizome significantly reduce systolic blood pressure and improve serum lipid profile in SHR fed a high-fat diet. However, the efficacy of purified polysaccharides, such as acidic polysaccharides, from Gastrodia rhizome as an antihypertensive agent has not been investigated. Therefore, we further purified the acidic polysaccharides from Gastrodia rhizome by ion-exchange chromatography on DEAE-Sepharose CL-6B, and evaluated the effects of acidic polysaccharides on anti-hypertensive effect and serum lipid levels in SHR fed a high-fat diet. Purified acidic polysaccharides consisted mainly of xylose, glucose, galacturonic acid, and glucuronic acid. The acidic polysaccharide fraction from Gastrodia rhizome markedly reduced systolic blood pressure in SHR fed a high-fat diet. Furthermore, the acidic polysaccharides positively regulated serum lipid levels in SHR. These results suggest that the acidic polysaccharides from Gastrodia rhizome might be excellent natural antihypertensive and anti-atherosclerosis agents due to their biological properties. These new findings help fill a critical gap between epidemiological observations and clinical studies on the antihypertensive benefits of acidic polysaccharides from Gastrodia rhizome. Further mechanism studies are needed to address this important issue.
Acknowledgments
This research was supported by Technology Development Program for Food, Ministry for Food, Agriculture, Forestry and Fisheries, Republic of Korea to H-D Hong and by Hyejeon College to K-I Kim.
References
- 1.Padmanabhan S., Paul L., Dominczak A.F. The pharmacogenomics of anti-hypertensive therapy. Pharmaceuticals. 2010;3:1779–1791. doi: 10.3390/ph3061779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peters P.G., Alessio H.M., Hagerman A.E., Ashton T., Nagy S., Wiley R.L. Short-term isometric exercise reduces systolic blood pressure in hypertensive adults: Possible role of reactive oxygen species. Int. J. Cardiol. 2006;110:199–205. doi: 10.1016/j.ijcard.2005.07.035. [DOI] [PubMed] [Google Scholar]
- 3.Roberts C.K., Barnard R.J. Effects of exercise and diet on chronic disease. J. Appl. Physiol. 2005;98:3–30. doi: 10.1152/japplphysiol.00852.2004. [DOI] [PubMed] [Google Scholar]
- 4.Galloway J.M. The epidemiology of atherosclerosis and its risk factors among Native Americans. Curr. Diab. Rep. 2002;2:274–81. doi: 10.1007/s11892-002-0095-1. [DOI] [PubMed] [Google Scholar]
- 5.Sacks F.M., Appel L.J., Moore T.J., Obarzanek E., Vollmer W.M., Svetkey L.P., Bray G.A., Vogt T.M., Cutler J.A., Windhauser M.M., et al. A dietary approach to prevent hypertension: A review of the Dietary Approaches to Stop Hypertension (DASH) study. Clin. Cardiol. 1999;22:III6–10. doi: 10.1002/clc.4960221503. [DOI] [PubMed] [Google Scholar]
- 6.Craig W.J. Nutrition concerns and health effects of vegetarian diets. Nutr. Clin. Pract. 2010;25:613–620. doi: 10.1177/0884533610385707. [DOI] [PubMed] [Google Scholar]
- 7.Xie J.T., Wu J.A., Mehendale S., Aung H.H., Yuan C.S. Anti-hyperglycemic effect of the polysaccharides fraction from American ginseng berry extract in ob/ob mice. Phytomedicine. 2004;11:182–187. doi: 10.1078/0944-7113-00325. [DOI] [PubMed] [Google Scholar]
- 8.Miyazawa N., Okazaki M., Ohga S. Antihypertensive effect of Pleurotus nebrodensis in spontaneously hypertensive rats. J. Oleo Sci. 2008;57:675–681. doi: 10.5650/jos.57.675. [DOI] [PubMed] [Google Scholar]
- 9.Hong H.D., Shim E.J., Kim K.I., Choi S.Y., Han C.K. Effect of Gastrodia elata Blume components on systolic blood pressure and serum lipid concentrations in spontaneously hypertensive rats fed high fat diet. J. Korean Soc. Food Sci. Nutr. 2007;36:174–179. [Google Scholar]
- 10.Tang W., Eisenbrand G. Chinese Drugs of Plant Origin. Chemistry, Pharmacology, and Use in Traditional and Modern Medicine. Springer-Verlag; Berlin Heidelberg, Germany: 1992. pp. 545–547. [Google Scholar]
- 11.Ding C.S., Shen Y.S., Li G., Wei Z., Wei F. Study of a glycoprotein from Gastrodia elata: Its effects of anticoagulation and antithrombosis. Zhongguo Zhong Yao Za Zhi. 2007;32:1060–1064. [PubMed] [Google Scholar]
- 12.Gutiérrez R.M.P. Orchids: A review of uses in traditional medicine, its phytochemistry and pharmacology. J. Med. Plants Res. 2010;4:592–638. [Google Scholar]
- 13.Hong Q.M., Shi S.S., Wang C., Wang S.C., Li X.L., Zhou X.J. Structural characterization of a glucan isolated from Gastrodia elata. Zhong Yao Cai. 2010;33:726–729. [PubMed] [Google Scholar]
- 14.Tong X.K., Qiu H., Zhang X., Shi L.P., Wang G.F., Ji F.H., Ding H.Y., Tang W., Ding K., Zuo J.P. WSS45, a sulfated alpha-D-glucan, strongly interferes with Dengue 2 virus infection in vitro. Acta Pharmacol. Sin. 2010;31:585–592. doi: 10.1038/aps.2010.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hayashi J., Sekine T., Deguchi S., Lin Q., Horie S., Tsuchiya S., Yano S., Watanabe K., Ikegami F. Phenolic compounds from Gastrodia rhizome and relaxant effects of related compounds on isolated smooth muscle preparation. Phytochemistry. 2002;59:513–539. doi: 10.1016/s0031-9422(02)00008-0. [DOI] [PubMed] [Google Scholar]
- 16.Kim H.J., Moon K.D., Lee D.S., Lee S.H. Ethyl ether fraction of Gastrodia elata Blume protects amyloid beta peptide-induced cell death. J. Ethnopharmacol. 2003;84:95–98. doi: 10.1016/s0378-8741(02)00290-8. [DOI] [PubMed] [Google Scholar]
- 17.Hong H.D., Kim Y.C., Keum I.K., Kim S.S., Kim K.I., Han C.K. Effect of Gastrodia rhizome fractions on serum lipid concentrations in rats fed with high fat diet. J. Korean Soc. Appl. Biol. Chem. 2005;48:370–374. [Google Scholar]
- 18.Xu X., Lu Y., Bie X. Protective effects of gastrodin on hypoxia-induced toxicity in primary cultures of rat cortical neurons. Planta Med. 2007;73:650–654. doi: 10.1055/s-2007-981523. [DOI] [PubMed] [Google Scholar]
- 19.Zhu H.B., Geng M.Y., Guan H.S., Zhang J.T. Antihypertensive effects of d-polymannuronic sulfate and its related mechanisms in renovascular hypertensive rats. Acta Pharmacol. Sin. 2000;21:727–732. [PubMed] [Google Scholar]
- 20.Hou W.C., Lee M.H., Hsu F.L., Lin Y.H. Inhibitory activities of semicarbazide-sensitive amine oxidase and angiotensin converting enzyme of pectin hydroxamic acid. J. Agric. Food Chem. 2003;51:6362–6366. doi: 10.1021/jf034463a. [DOI] [PubMed] [Google Scholar]
- 21.Matsui T., Zhu X.L., Shiraishi K., Ueki T., Noda Y., Matsumoto K. Antihypertensive effect of salt-free soy sauce, a new fermented seasoning, in spontaneously hypertensive rats. J. Food Sci. 2010;75:H129–H134. doi: 10.1111/j.1750-3841.2010.01599.x. [DOI] [PubMed] [Google Scholar]
- 22.Dubois M., Gilles K.A., Dong Q., Fang J., Li X. Colorimetric method for determination of sugars and related substance. Anal. Chem. 1956;28:350–356. [Google Scholar]
- 23.Thetsrimuang C., Khammuang S., Sarnthima R. Antioxidant activity of crude polysaccharides from edible fresh and dry mushroom fruiting bodies of Lentinus sp. strain RJ-2. Int. J. Pharmacol. 2011;7:58–65. [Google Scholar]
- 24.Han C.K., Lee O.H., Kim K.I., Park J.M., Kim Y.C., Lee B.Y. Effect of powder, 50% ethanol and hot water extracts of Gastrodiae Rhizoma on serum lipids and blood pressure in SHR fed high-fat diet. J. Korean Soc. Food Sci. Nutr. 2003;32:1095–1101. [Google Scholar]
- 25.Rhyu M.R., Kim E.Y., Han J.S. Antihypertensive effect of the soybean paste fermented with the fungus Monascus. Int. J. Food Sci. Technol. 2002;37:585–588. [Google Scholar]
- 26.Lee Y.K., Woo M.H., Kim C.H., Kim Y., Lee S.H., Jeong B.S., Chang H.W., Son J.K. Two new benzofurans from Gastrodia elata and their DNA topoisomerases I and II inhibitory activities. Planta Med. 2007;73:1287–1291. doi: 10.1055/s-2007-981619. [DOI] [PubMed] [Google Scholar]
- 27.Lee B.Y., Choi H.S., Hwang J.B. Analysis of food components of Gratrodiae Rhizoma and changes in several characteristics at the various drying conditions. Korean J. Food Sci. Technol. 2002;34:37–42. [Google Scholar]