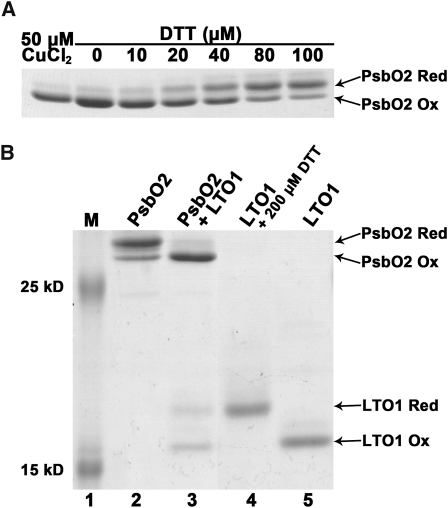
Figure 9.
The Thioredoxin-Like Domain of LTO1 Catalyzes Disulfide Bond Formation in PsbO2.
Recombinant proteins (PsbO2 and the thioredoxin-like domain of LTO1) were treated with AMS, separated by nonreducing SDS-PAGE (15%), and visualized by Coomassie blue staining. The positions of reduced (PsbO2 Red) and oxidized (PsbO2 Ox) forms of PsbO2 and reduced (LTO1 Red) and oxidized (LTO1 Ox) forms of LTO1 are indicated by arrows. AMS is an alkylating reagent, and AMS treatment of exposed thiols in PsbO2 and LTO1 results in an increased molecular mass of the alkylated molecules. Only reduced PsbO2 or LTO1 reacts with AMS.
(A) DTT-dependent reduction of the disulfide in PsbO2. Lane 1, recombinant PsbO2 was fully oxidized by incubation with 50 μM CuCl2. Lanes 2 to 7, air-oxidized PsbO2 was reduced by 1 h incubation with increasing concentrations of DTT.
(B) LTO1-dependent oxidation of PsbO2 sulfhydryls. Prestained protein ladder (Fermentas) was used in lane 1. Lane 2, reduced PsbO2 after removal of DTT; lane 3, reduced PsbO2 (10 μM) mixed with oxidized LTO1 thioredoxin-like domain (16 μM); lane 4, DTT-reduced LTO1 thioredoxin-like domain; and lane 5, air-oxidized LTO1. Quantification using ImageJ software indicates that 72% of reduced PsbO2 (7.2 μM) becomes oxidized, whereas 44% of oxidized LTO1 (7.04 μM) is converted to its reduced form.