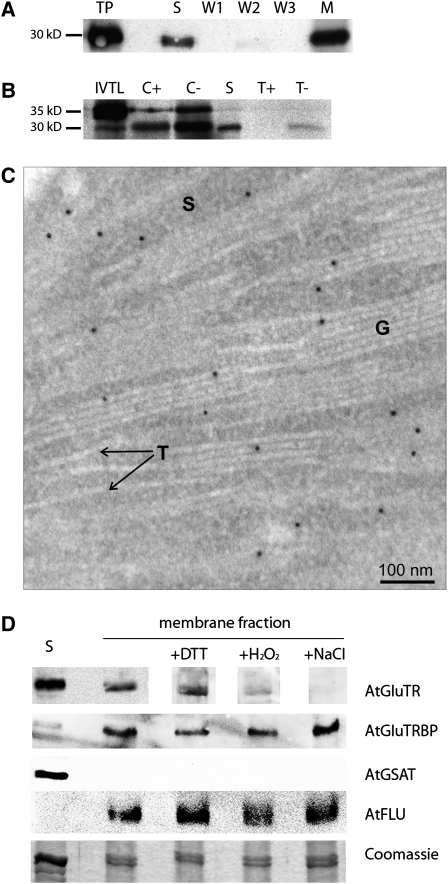
Figure 2.
At-GluTRBP Is Localized in the Chloroplast and Attached to the Thylakoid Membrane.
(A) In immunoblot analysis the 30-kD mature At-GluTRBP was detected in the stroma fraction (S) as well as in the membrane fraction (M) of isolated chloroplasts (W1 to W3, serial membrane washing fractions). An Arabidopsis total protein extract (TP) was used as control.
(B) In vitro full-length [35S]-labeled At-GluTRBP (IVTL) was incubated with isolated pea chloroplasts, resulting in two proteins with apparent molecular masses of 35.5 and 30.7 kD (C−). After thermolysin treatment of the chloroplasts (C+), only the mature 30.7-kD protein was protected against proteolytic digestion and found in the stroma fraction (S) as well as bound to membranes (T−). Thermolysin treatment of the membrane fraction (T+) digested At-GluTRBP.
(C) Immunogold localization of GluTRBP in chloroplasts of Arabidopsis leaves. GluTRBP is detected exclusively in the stroma (S) and the grana stacks (G) but not in the envelope or stroma thylakoids (T). See Supplemental Data Set 1 online for more pictures (e.g., for confirmation that GluTRBP is missing on envelope membranes and for additional controls). Magnification ×89,000.
(D) Localization of proteins within Arabidopsis chloroplasts. Chloroplasts from 4-week-old Arabidopsis plants were separated into stroma (S) and membrane fractions. Membranes were washed additionally with 10 mM DTT, 10 mM H2O2, or 4 M NaCl. GluTR, GluTRBP, GSAT, and FLU were detected by immunoblot analysis using specific antibodies. Parts of a Coomassie blue–stained 12% SDS-polyacrylamide gel were used as a loading control.