Abstract
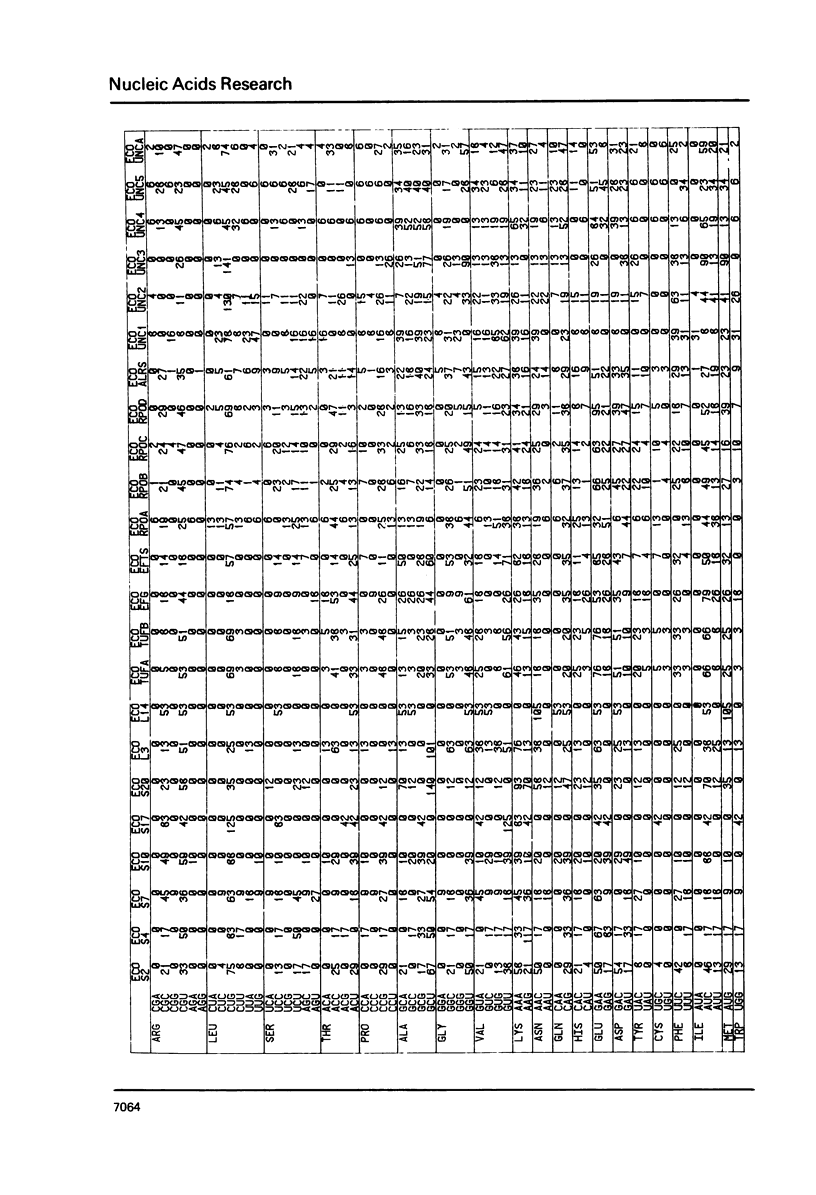
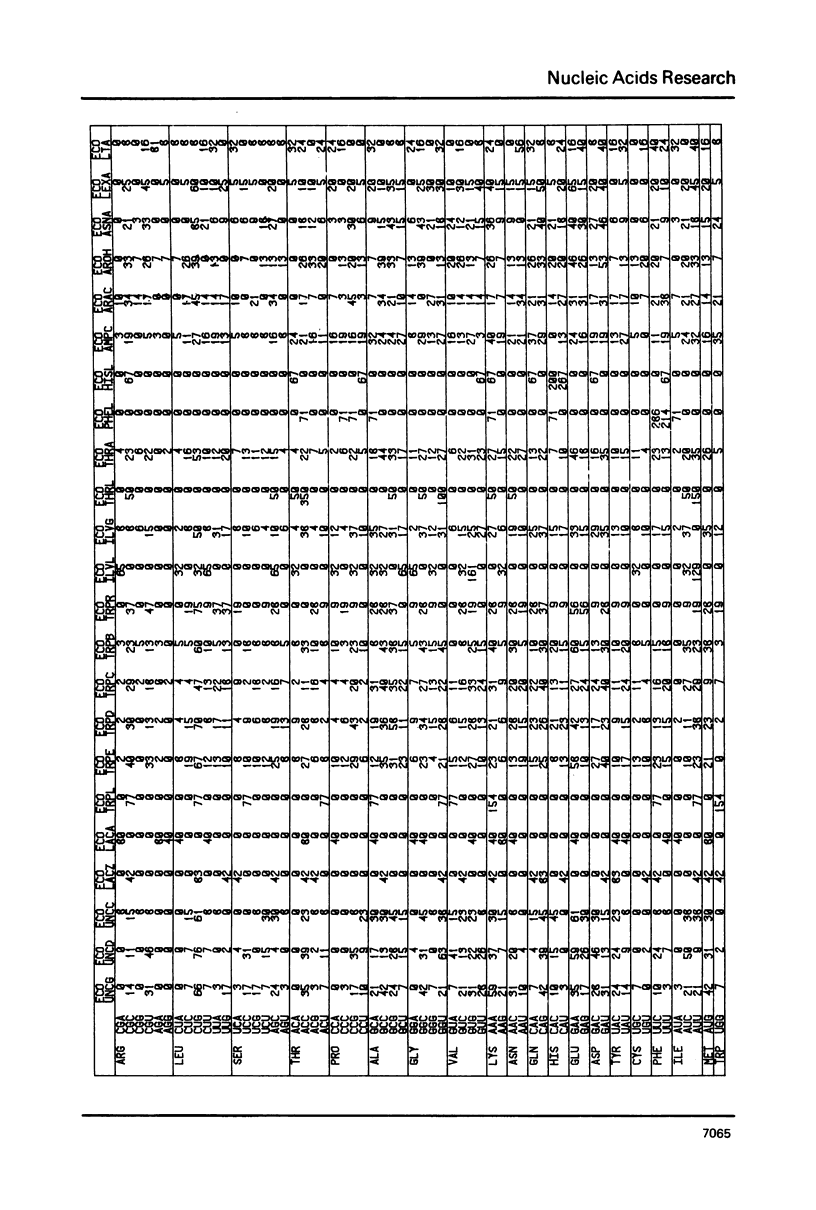
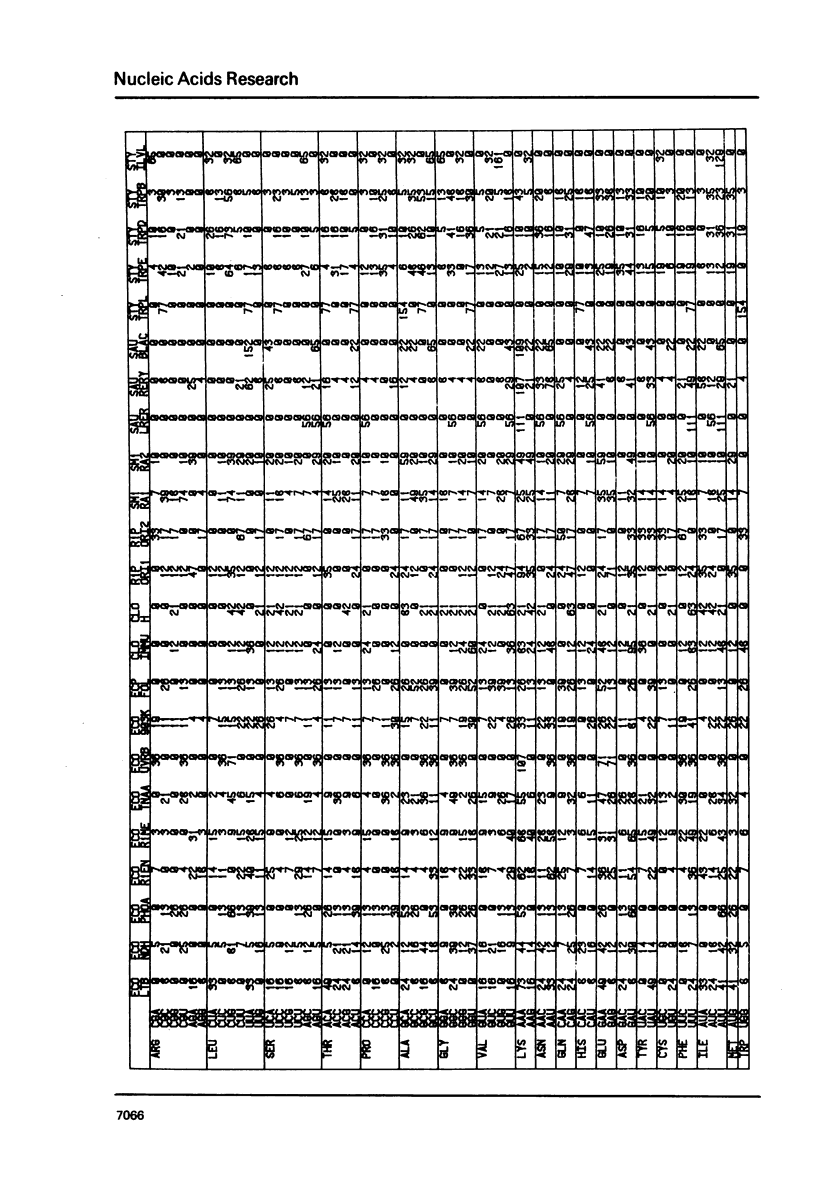
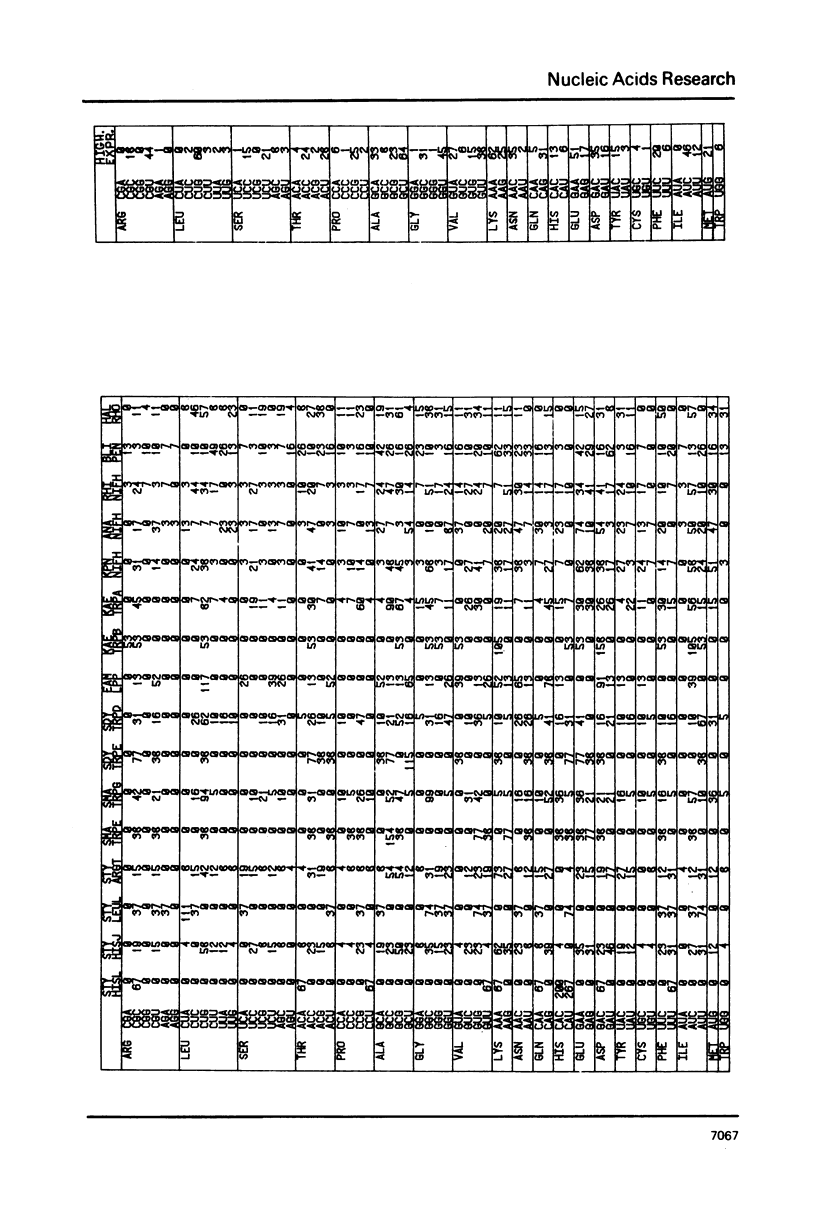
The nucleic acid sequence bank now contains over 600 protein coding genes of which 107 are from prokaryotic organisms. Codon frequencies in each new prokaryotic gene are given. Analysis of genetic code usage in the 83 sequenced genes of the Escherichia coli genome (chromosome, transposons and plasmids) is presented, taking into account new data on gene expressivity and regulation as well as iso-tRNA specificity and cellular concentration. The codon composition of each gene is summarized using two indexes: one is based on the differential usage of iso-tRNA species during gene translation, the other on choice between Cytosine and Uracil for third base. A strong relationship between codon composition and mRNA expressivity is confirmed, even for genes transcribed in the same operon. The influence of codon use of peptide elongation rate and protein yield is discussed. Finally, the evolutionary aspect of codon selection in mRNA sequences is studied.
Full text
PDF













Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennetzen J. L., Hall B. D. Codon selection in yeast. J Biol Chem. 1982 Mar 25;257(6):3026–3031. [PubMed] [Google Scholar]
- Bergmann J. E., Lodish H. F. A kinetic model of protein synthesis. Application to hemoglobin synthesis and translational control. J Biol Chem. 1979 Dec 10;254(23):11927–11937. [PubMed] [Google Scholar]
- Burton Z., Burgess R. R., Lin J., Moore D., Holder S., Gross C. A. The nucleotide sequence of the cloned rpoD gene for the RNA polymerase sigma subunit from E coli K12. Nucleic Acids Res. 1981 Jun 25;9(12):2889–2903. doi: 10.1093/nar/9.12.2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadaban M. J. Regulation of the regulatory gene for the arabinose pathway, araC. J Mol Biol. 1976 Jul 5;104(3):557–566. doi: 10.1016/0022-2836(76)90120-0. [DOI] [PubMed] [Google Scholar]
- Chamberlin M. J. The selectivity of transcription. Annu Rev Biochem. 1974;43(0):721–775. doi: 10.1146/annurev.bi.43.070174.003445. [DOI] [PubMed] [Google Scholar]
- Chavancy G., Garel J. P. Does quantitative tRNA adaptation to codon content in mRNA optimize the ribosomal translation efficiency? Proposal for a translation system model. Biochimie. 1981 Mar;63(3):187–195. doi: 10.1016/s0300-9084(81)80192-7. [DOI] [PubMed] [Google Scholar]
- Dennis P. P. Transcription patterns of adjacent segments on the chromosome of Escherichia coli containing genes coding for four 50S ribosomal proteins and the beta and beta' subunits of RNA polymerase. J Mol Biol. 1977 Oct 5;115(4):603–625. doi: 10.1016/0022-2836(77)90105-x. [DOI] [PubMed] [Google Scholar]
- DiRienzo J. M., Nakamura K., Inouye M. The outer membrane proteins of Gram-negative bacteria: biosynthesis, assembly, and functions. Annu Rev Biochem. 1978;47:481–532. doi: 10.1146/annurev.bi.47.070178.002405. [DOI] [PubMed] [Google Scholar]
- Fiers W., Grosjean H. On codon usage. Nature. 1979 Jan 25;277(5694):328–328. doi: 10.1038/277328a0. [DOI] [PubMed] [Google Scholar]
- Furano A. V. Content of elongation factor Tu in Escherichia coli. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4780–4784. doi: 10.1073/pnas.72.12.4780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garel J. P., Mandel P., Chavancy G., Daillie J. Functional adaptation of tRNAs to fibroin biosynthesis in the silkgland of Bombyx mori L. FEBS Lett. 1970 May 1;7(4):327–329. doi: 10.1016/0014-5793(70)80196-x. [DOI] [PubMed] [Google Scholar]
- Gay N. J., Walker J. E. The atp operon: nucleotide sequence of the promoter and the genes for the membrane proteins, and the delta subunit of Escherichia coli ATP-synthase. Nucleic Acids Res. 1981 Aug 25;9(16):3919–3926. doi: 10.1093/nar/9.16.3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gay N. J., Walker J. E. The atp operon: nucleotide sequence of the region encoding the alpha-subunit of Escherichia coli ATP-synthase. Nucleic Acids Res. 1981 May 11;9(9):2187–2194. doi: 10.1093/nar/9.9.2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert W., Müller-Hill B. Isolation of the lac repressor. Proc Natl Acad Sci U S A. 1966 Dec;56(6):1891–1898. doi: 10.1073/pnas.56.6.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouy M., Grantham R. Polypeptide elongation and tRNA cycling in Escherichia coli: a dynamic approach. FEBS Lett. 1980 Jun 30;115(2):151–155. doi: 10.1016/0014-5793(80)81155-0. [DOI] [PubMed] [Google Scholar]
- Grantham R., Gautier C., Gouy M. Codon frequencies in 119 individual genes confirm consistent choices of degenerate bases according to genome type. Nucleic Acids Res. 1980 May 10;8(9):1893–1912. doi: 10.1093/nar/8.9.1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grantham R., Gautier C., Gouy M., Jacobzone M., Mercier R. Codon catalog usage is a genome strategy modulated for gene expressivity. Nucleic Acids Res. 1981 Jan 10;9(1):r43–r74. doi: 10.1093/nar/9.1.213-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosjean H. J., de Henau S., Crothers D. M. On the physical basis for ambiguity in genetic coding interactions. Proc Natl Acad Sci U S A. 1978 Feb;75(2):610–614. doi: 10.1073/pnas.75.2.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosjean H., Sankoff D., Jou W. M., Fiers W., Cedergren R. J. Bacteriophage MS2 RNA: a correlation between the stability of the codon: anticodon interaction and the choice of code words. J Mol Evol. 1978 Dec 29;12(2):113–119. doi: 10.1007/BF01733262. [DOI] [PubMed] [Google Scholar]
- Gudas L. J., Pardee A. B. DNA synthesis inhibition and the induction of protein X in Escherichia coli. J Mol Biol. 1976 Mar 15;101(4):459–477. doi: 10.1016/0022-2836(76)90240-0. [DOI] [PubMed] [Google Scholar]
- Ikemura T. Correlation between the abundance of Escherichia coli transfer RNAs and the occurrence of the respective codons in its protein genes. J Mol Biol. 1981 Feb 15;146(1):1–21. doi: 10.1016/0022-2836(81)90363-6. [DOI] [PubMed] [Google Scholar]
- Ikemura T. Correlation between the abundance of Escherichia coli transfer RNAs and the occurrence of the respective codons in its protein genes: a proposal for a synonymous codon choice that is optimal for the E. coli translational system. J Mol Biol. 1981 Sep 25;151(3):389–409. doi: 10.1016/0022-2836(81)90003-6. [DOI] [PubMed] [Google Scholar]
- Keller E. B., Calvo J. M. Alternative secondary structures of leader RNAs and the regulation of the trp, phe, his, thr, and leu operons. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6186–6190. doi: 10.1073/pnas.76.12.6186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawther R. P., Calhoun D. H., Adams C. W., Hauser C. A., Gray J., Hatfield G. W. Molecular basis of valine resistance in Escherichia coli K-12. Proc Natl Acad Sci U S A. 1981 Feb;78(2):922–925. doi: 10.1073/pnas.78.2.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawther R. P., Nichols B., Zurawski G., Hatfield G. W. The nucleotide sequence preceding and including the beginning of the ilvE gene of the ilvGEDA operon of Escherichia coli K12. Nucleic Acids Res. 1979 Dec 20;7(8):2289–2301. doi: 10.1093/nar/7.8.2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl L., Post L., Zengel J., Gilbert S. F., Strycharz W. A., Nomura M. Mapping of ribosomal protein genes by in vitro protein synthesis using DNA fragments of lambdafus3 transducing phage DNA as templates. J Biol Chem. 1977 Oct 25;252(20):7365–7383. [PubMed] [Google Scholar]
- Little R., Fiil N. P., Dennis P. P. Transcriptional and post-transcriptional control of ribosomal protein and ribonucleic acid polymerase genes. J Bacteriol. 1981 Jul;147(1):25–35. doi: 10.1128/jb.147.1.25-35.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse D. E., Mosteller R. D., Yanofsky C. Dynamics of synthesis, translation, and degradation of trp operon messenger RNA in E. coli. Cold Spring Harb Symp Quant Biol. 1969;34:725–740. doi: 10.1101/sqb.1969.034.01.082. [DOI] [PubMed] [Google Scholar]
- Pedersen S., Bloch P. L., Reeh S., Neidhardt F. C. Patterns of protein synthesis in E. coli: a catalog of the amount of 140 individual proteins at different growth rates. Cell. 1978 May;14(1):179–190. doi: 10.1016/0092-8674(78)90312-4. [DOI] [PubMed] [Google Scholar]
- Post L. E., Nomura M. DNA sequences from the str operon of Escherichia coli. J Biol Chem. 1980 May 25;255(10):4660–4666. [PubMed] [Google Scholar]
- Post L. E., Nomura M. Nucleotide sequence of the intercistronic region preceding the gene for RNA polymerase subunit alpha in Escherichia coli. J Biol Chem. 1979 Nov 10;254(21):10604–10606. [PubMed] [Google Scholar]
- Post L. E., Strycharz G. D., Nomura M., Lewis H., Dennis P. P. Nucleotide sequence of the ribosomal protein gene cluster adjacent to the gene for RNA polymerase subunit beta in Escherichia coli. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1697–1701. doi: 10.1073/pnas.76.4.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post L. E., Strycharz G. D., Nomura M., Lewis H., Dennis P. P. Nucleotide sequence of the ribosomal protein gene cluster adjacent to the gene for RNA polymerase subunit beta in Escherichia coli. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1697–1701. doi: 10.1073/pnas.76.4.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose J. K., Yanofsky C. Interaction of the operator of the tryptophan operon with repressor. Proc Natl Acad Sci U S A. 1974 Aug;71(8):3134–3138. doi: 10.1073/pnas.71.8.3134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson K. E. Genetic relatedness in the family Enterobacteriaceae. Annu Rev Microbiol. 1976;30:327–349. doi: 10.1146/annurev.mi.30.100176.001551. [DOI] [PubMed] [Google Scholar]
- Saraste M., Gay N. J., Eberle A., Runswick M. J., Walker J. E. The atp operon: nucleotide sequence of the genes for the gamma, beta, and epsilon subunits of Escherichia coli ATP synthase. Nucleic Acids Res. 1981 Oct 24;9(20):5287–5296. doi: 10.1093/nar/9.20.5287. [DOI] [PMC free article] [PubMed] [Google Scholar]


