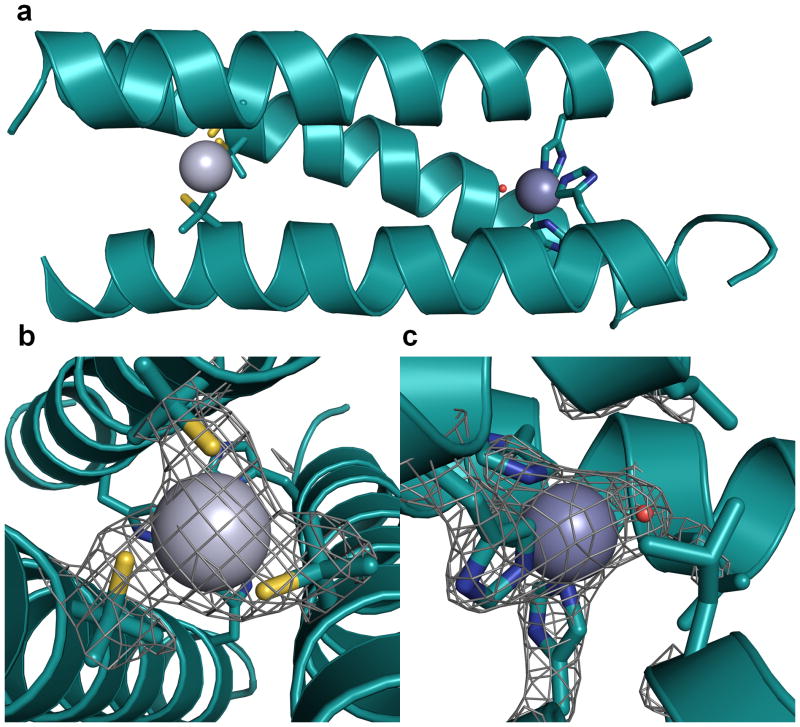
Figure 1. Ribbon diagrams of the [Hg(II)]S[Zn(II)(H2O/OH )]N(CSL9PenL23H)3n+ parallel 3SCC (one of two different 3-helix bundles present in the asymmetric unit) at pH 8.5.
Shown are the main chain atoms represented as helical ribbons (cyan) and the Pen and His side chains in stick form (sulphur = yellow, nitrogen = blue, oxygen = red). a, One of two trimers found in the asymmetric unit of the crystal structure. b, a top down view of the structural trigonal thiolate site, Hg(II)S3, confirming the proposed structure of Hg(II) in Cys-containing TRI peptides.17 This metal site should mimic well the structural site in the metalloregulatory protein MerR.47 c, a side view of the tetrahedral catalytic site, Zn(II)N3O, which closely mimics carbonic anhydrase and matrix metalloproteinase active sites.1 All figures are shown with 2Fo-Fc electron density contoured at 1.5 σ overlaid.