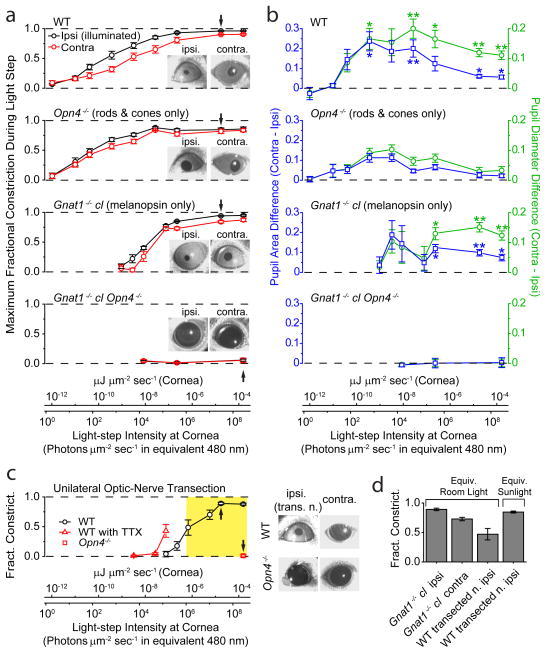
Figure 6.
Simultaneous direct (ipsilateral) and consensual (contralateral) PLRs to unilateral illumination for different mouse genotypes in situ. 2-min of 505-nm LED light in a–c. PLR in ipsilateral, illuminated eye was measured at peak during this 2-min period, with contralateral PLR measured simultaneously. For a and b, conversion of light intensities into equivalent 480-nm photons applies strictly only to Gnat1−/− cl, but not to WT or Opn4−/− genotypes (which involved also rod/cone signals), because it required action spectrum of melanopsin. For this reason, light intensities are also given in the general unit of μJ μm−2 s−1 (same as μW μm−2). a, Average step intensity-response (I-R) relations for WT, Opn4−/−, Gnat1−/− cl, and Gnat1−/− cl Opn4−/− mice. PLR expressed as MFC (Maximum Fractional Constriction), where MFC = 1 (Normalized Pupil Area in Light) = 1 − (Pupil Area in Light/Pupil Area in Darkness). Inset shows exemplary bilateral PLRs at an intensity indicated by arrow on I-R relations. Number of animals were 7 (WT), 7 (Opn4−/−), 5 (Gnat1−/− cl) and 3 (Opn4−/− Gnat1−/− cl). b, Ipsi/contralateral difference in normalized pupil area and diameter. For area, this difference is simply the difference between the ipsi- and contralateral values in a. For diameter, normalized diameter is (Normalized Area)1/2 = (1- MFC)1/2, calculable from data in a; the ipsi/contralateral difference values can then evaluated (see Methods for significance of diameter). The area or diameter difference at a given intensity was then averaged over all animals of a given genotypic group. “**” indicates p<0.01, and “*” indicates p<0.05, when a WT or Gnat1−/− cl value is compared to corresponding Opn4−/− value by two-samples t-test. c, Direct (ipsilateral) PLR of eye with transected optic nerve to isolate the intrinsic component. WT mice in the absence or presence of TTX (5 μl of 600-μM TTX in water administered on the cornea) (same set of 5 animals), and Opn4−/− mice (4 animals). Images on right show exemplary bilateral PLRs (without TTX) at an intensity indicated by arrow on corresponding I-R relations. Opn4−/− animals with transected optic nerve gave essentially no PLR. Yellow shaded area indicates light-intensity range from room light (with a minimum measured as 1.0 × 10−6 μW μm−2, or 1.1 × 106 equivalent 480-nm photons μm−2 s−1) to direct sunlight (measured as 3.7 × 10−4 μW μm−2, or 3.9 × 108 equivalent 480-nm photons μm−2 s−1) (Methods). d, Pupil constriction triggered by white light (400–650-nm bandpass filter; Xe lamp), matched in power to average common room light and ambient sunlight (Methods) (2.2 × 10−6 and 4.2 × 10−5 μW μm−2, respectively), for Gnat1−/− cl without optic-nerve transaction (6 animals) and WT mice with optic-nerve transaction (5 animals). All light rendered adirectional with Ganzfeld diffusing sphere. Pupils video-recorded under dim infrared light that did not activate any photoreceptors. All error bars are S.E.M.