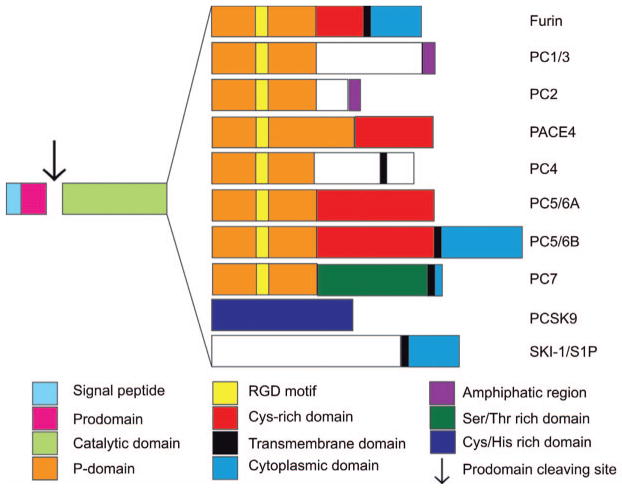
Figure 1. Proprotein convertases structural domains.
Structurally, each PC possess five characteristic domains which are; (i) the signal peptide at the N-terminal extremity, responsible for the entry of the enzyme into the secretory pathway; (ii) the pro-domain which directs the suitable folding of the enzyme (chaperone), but is also an intramolecular inhibitor keeping the enzyme in its inactive form until it reaches a compartment with the appropriate calcium concentration and pH to be autoprocessed, allowing its removal; (iii) the catalytic domain, with high homology among the PC family, containing the catalytic triad composed of an Asp, His and a Ser, responsible for the interaction and the cleavage of the substrate’s multi-basic residues; (iv) the P-domain plays a key role in enzyme stability, calcium and pH dependence; and finally (v) a C-terminal domain that varies for each PC and may include transmembrane, cytoplasmic, amphipathic, serine/threonine or cysteine-histidine rich domains, contributing to each PCs unique cellular localization features.