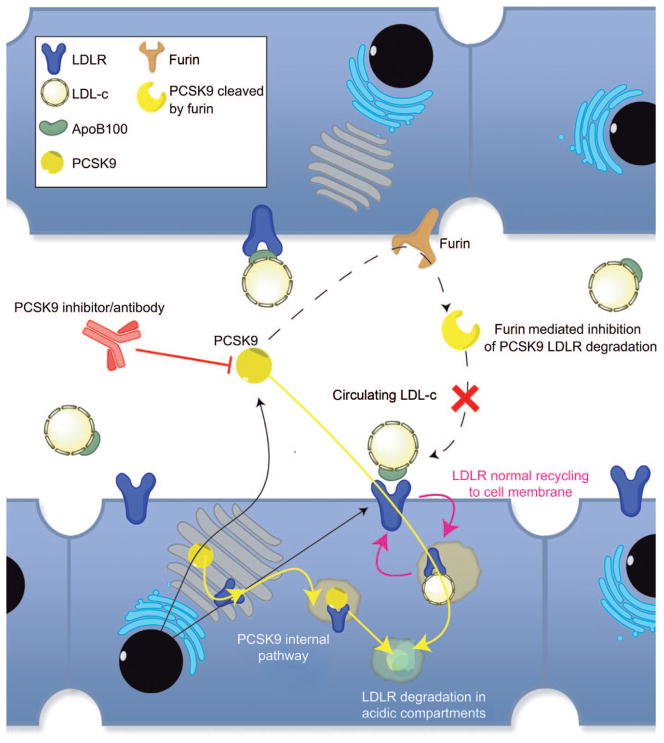
Figure 3. PCSK9 pharmacological inhibition strategy.
From the blood circulation, cholesterol cellular uptake of LDL-c particles within the liver is mediated by the recognition of ApoB100 by the hepatocyte LDLR. This receptor-mediated endocytosis of the LDL-c leads to a decrease in plasma concentrations PCSK9, which is a secreted protein, is capable of binding LDLR (and VLDLR) (52) by its EGF-A domain (53) and mediates its degradation through late endosomal or lysosomal digestion PCSK9 can also mediate the degradation of de novo synthesized receptors by intercepting them before they even reach the plasma membrane (54) PCSK9 in circulation can be inactivated by furin or PC5/6A cleavage, which indicates a new regulation mechanism to control levels of active PCKS9. Interesting findings also show that some PCSK9 gain-of-function mutation are due to the removal of such inactivating cleavage site, leading to hypercholesterolemia (55).