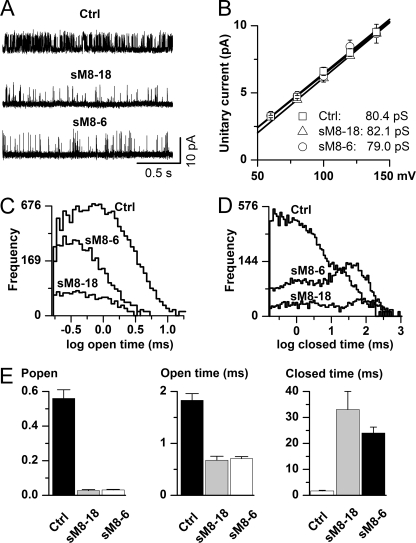
FIGURE 7.
Ion channel mechanism underlying inhibition of TRPM8 channel by short TRPM8 isoforms. A, representative examples of single-channel activity recorded in the cell-attached configuration in membrane patches held at 120 mV relative to the cell resting potential. Less frequent channel openings were evident with the sM8-18 (n = 5) or sM8-6 (n = 10) isoform coexpressed with the full-length TRPM8 protein (Ctrl; n = 6). B, the isoforms did not alter single-channel conductance, suggesting that short TRPM8 isoforms do not cause any hindrance of the ion permeation pathway. This analysis was restricted to positive potentials only because openings were brief and likely incompletely resolved in the negative range (21). C and D, comparisons of the open (C) and closed (D) interval distributions constructed using logarithmic binning (20 bins per decade) after executing the event search algorithm of the pCLAMP 9 program. Events briefer than 0.16 ms were ignored as poorly resolvable (21). Note that the main changes occurred in the long closed component domain. In parallel, the short isoforms significantly reduced the proportion of short closings. E, mean data showing reduction in the channel Po (left panel) accompanied by some reduction in the mean open time (middle panel) and a dramatic increase in the mean closed time (right panel) with either isoform. Statistical tests using analysis of variance with the Tukey-Kramer multiple-comparison test revealed significant differences between mean values for all measured parameters (p < 0.0001 in all cases), with both isoforms showing differences from the control (p < 0.001 in all cases) but not between themselves (p > 0.05 in all cases).