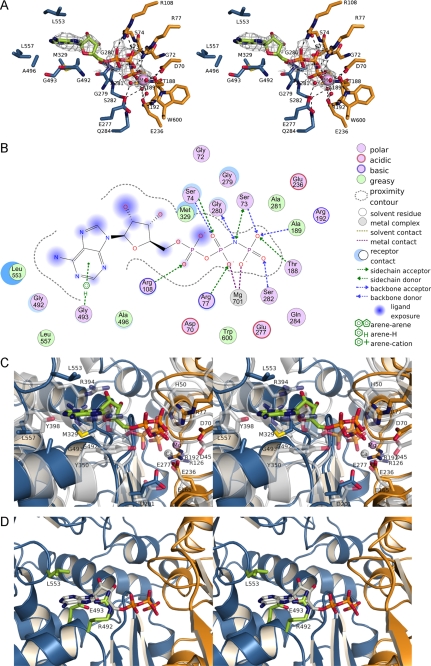
FIGURE 7.
Substrate binding to TgNTPDases. A, AMPPNP bound to the active site of TgNTPDase3ΔCC. Residues involved in the hydrolysis of ATP are shown as sticks and are colored as described in the legend to Fig. 1. Water molecules are not present in the crystal structure and were modeled according to the active site of RnNTPDase2 (41). The omit electron density map (Fo − Fc × ϕc, contoured at 2 σ) of AMPPNP is shown in gray. B, scheme of the interactions of Mg2+-AMPPNP in the active site of TgNTPDase3ΔCC. The size of the blue clouds indicates the solvent accessibility of the ligand atoms. The strength of the interactions to the ligands is indicated by a blue shadow behind the residue. C, superposition of RnNTPDase2 (gray) and TgNTPDase3ΔCC (orange/blue; bound AMPPNP green, Mg2+ purple) on all residues. D, superposition of AMPPNP (gray) from the TgNTPDase3ΔCC-Mg2+-AMPPNP complex on TgNTPDase1ΔCC with putative base binding residues colored in green.