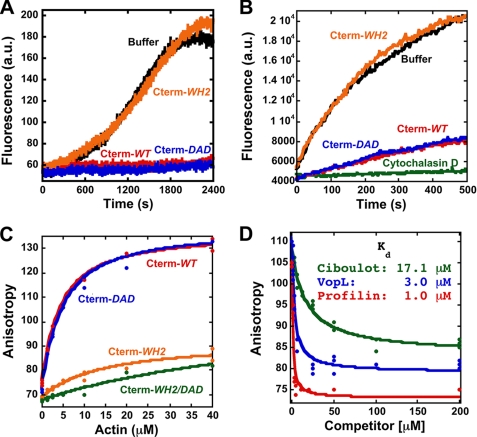
FIGURE 6.
The C terminus of FMNL3 contains a WH2-like sequence whose mutation disrupts monomer binding and inhibition of filament elongation. A, pyrene-actin polymerization assays using 2 μm actin monomers (5% pyrene) and 16 μm concentrations of the indicated Cterm constructs. B, filament elongation assays using 3.1 μm polymerized actin, 1 μm actin monomers (25% pyrene), and 8 μm concentrations of the indicated Cterm constructs. C, direct binding assays using polarization anisotropy of 20 nm concentrations of the indicated fluorescein-labeled Cterm constructs and increasing concentrations of latrunculin B-bound actin monomers in polymerization buffer. D, a competition binding assay with FMNL3 and increasing concentrations of Profilin or WH2 peptides Ciboulot or VopL. Assays were performed with 5 μm latrunculin B-bound actin monomers and 20 nm FL-Cterm-WT in polymerization buffer.