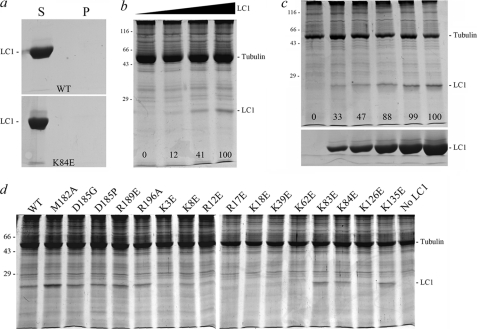
FIGURE 9.
Axoneme binding activity of mutant LC1 proteins. a, the supernatants (S) and pellets (P) for wild-type and K84E mutant LC1 spun in a microcentrifuge in the absence of axonemes. This panel demonstrates that monomeric LC1 does not sediment during centrifugation. b illustrates the dose-dependent binding of LC1 to axonemes from the outer armless mutant oda6. The numbers on the image indicate the relative amounts of LC1 bound to the axoneme sample (0.83 mg/ml) as the LC1 concentration was increased from 0 to 0.18, 0.94, and 1.88 mg/ml. c, increasing amounts of D185P LC1 (0, 0.07, 0.14, 0.28, 0.42, and 0.7 mg/ml final concentration) were added to dynein-depleted wild-type axonemes (0.7 mg/ml). The pellets from the binding assay are shown in the top panel, and equivalent loadings of the corresponding supernatants are shown in the lower panel. The numbers on the upper panel indicate the relative amounts of LC1 bound to a fixed amount of axonemes and demonstrate that the binding becomes saturated. d illustrates the effects of individual point mutations on LC1-axoneme binding; only pellets from the binding assay are shown. All assays contained the same concentration of both axonemal (0.9 mg/ml) and LC1 (0.56 mg/ml) proteins except for M182A (0.81 mg/ml). Several of the Lys → Glu mutations severely affected the ability of LC1 to bind axonemes, whereas the more C-terminal mutations did not: these data are quantified in Tables 1 and 2. All samples were separated in 12.5% SDS-polyacrylamide gels and stained with Coomassie Blue.