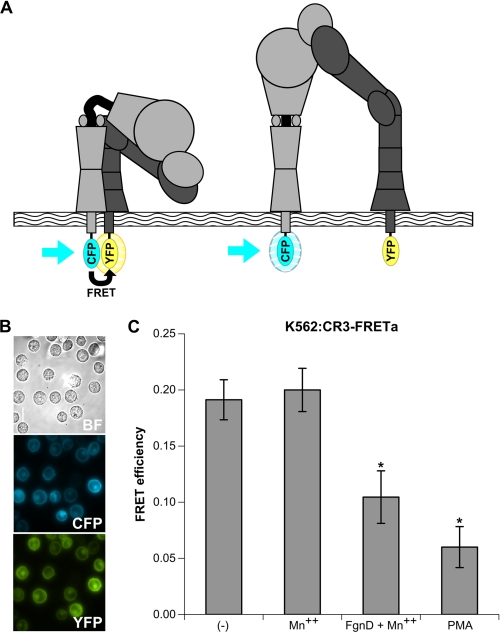
FIGURE 2.
The use of FRET to report integrin activation; development of a K562 cell line that stably expresses the CR3-FRET activation reporter; loss of FRET in activation reporter with native ligand. A, the integrin activation FRET reporter is shown. In the inactive, bent conformation (left), the cytoplasmic domains of the α and β subunits are closely apposed, allowing for efficient FRET between mCFP and mYFP fused to these C termini. In the active, extended conformation (right), the α and β subunits lose their interactions in the tailpiece leading to a separation greater than 100 Å between the mCFP and mYFP, thereby abolishing FRET. See also supplemental Figs. S1 and S2 for reporter construction and functional characterization. B, shown are bright field (BF), CFP, and YFP images of the K562 cell line stably expressing the CR3-FRET activation construct (K562:CR3-FRETa) acquired with a Nikon Eclipse TE2000-U epifluorescence microscope coupled to a CoolSNAP HQ CCD camera using NIS elements software. Nikon CFP HQ and YFP HQ cubes without emission filters were used for CFP and YFP imaging, respectively. Cells were visualized at 37 °C in L-15 medium with 2 mg/ml glucose. A xenon lamp was used to illuminate the cells through a 33-mm ND4 filter, and the cells were viewed through a 60× oil immersion Plan APO objective lens. Exposure time was 500 ms for both CFP and YFP. C, FRET efficiencies: basal FRET, non-activating treatment with 1 mm Mn2+ FRET, activation with 1 mm Mn2+ plus 25 μg/ml FgnD or 20 nm phorbol 12-myristate 13-acetate (PMA). Each sample represents analysis of at least 25 cells in three separate experiments. *, p < 0.01.