Figure 5.
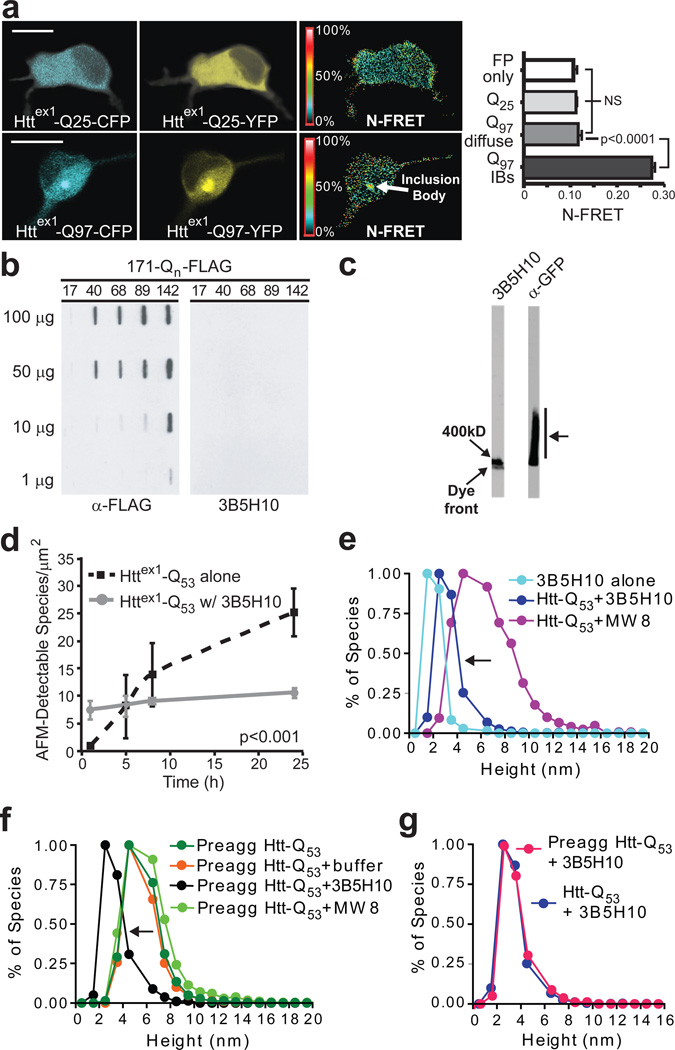
3B5H10 does not recognize large oligomers of mHtt. (a) N-FRET (mean±s.d.) from cortical neurons was high in IBs formed from Httex1-Q97-CFP and Httex1-Q97-YFP (Q97 IBs) but low in regions of neurons with diffuse mHtt (Q97 diffuse) and not significantly different than in neurons with Httex1-Q25-CFP and Httex1-Q25-YFP (Q25) or CFP and YFP (FP only). Scale bar=10 µm. (b) HEK293 extracts containing Htt-171-(Q17, Q40, Q68, Q89, or Q142)-FLAG were loaded on a 0.20-µm membrane slot-blot and probed with α-FLAG or 3B5H10. α-FLAG revealed submicroscopic aggregates of Htt-171-(Qn)-FLAG that were unrecognizable by 3B5H10. (c) Agarose gel electrophoresis of extracts from PC12 cells stably expressing truncated (no polyproline) Httex1-Q103-GFP were blotted with α-GFP or 3B5H10. While oligomeric Httex1-Q103-GFP was present (arrow), 3B5H10 only stained the dye front, which represents monomer and possibly small oligomers. (d) 3B5H10 prevents mHtt aggregation detected by AFM. Httex1-Q53 aggregation was triggered with or without 3B5H10. (e) AFM-detectable species observed with 3B5H10 alone or with 3B5H10 or MW8 added to monomeric Httex1-Q53. 3B5H10 addition to htt stabilizes a 2–3 nm globular species, consistent with the size of a monomeric htt:antibody complex. (f) AFM-detectable species observed with 3B5H10, MW8, buffer, or nothing added to pre-aggregated oligomers of Httex1-Q53. 3B5H10 had similar effects when added to pre-formed fibrils (Supplementary Fig. 10 online). (g) The final size of AFM-detectable species when 3B5H10 is added to monomeric Httex1-Q53 (Fig. 5e) or pre-aggregated oligomers (Fig. 5f) is statistically indistinguishable (Spearman’s rank correlation coefficient). This size is most consistent with a monomeric htt:antibody complex.