Table 6.
Expanded Reaction Scope for Asymmetric Catalytic Diazoester Aldol.
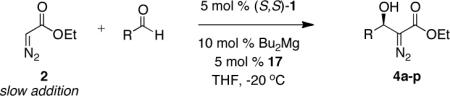
| Entrya | RCHO | Product | Time (h) | Yield (%)b | ee (%)c |
|---|---|---|---|---|---|
| 1 | Ph 3a | 4a | 18 | 92 | 95 |
| 2d | Ph 3a | 4a | 24 | 95 | −95 |
| 3 | m-CH3OC6H4 3b | 4b | 18 | 83 | 90 |
| 4 | p-CH3OC6H4 3c | 4c | 18 | 70 | 87 |
| 5 | o-CIC6H4 3d | 4d | 18 | 91 | 89 |
| 6 | m-FC6H4 3e | 4e | 24 | 78 | 91 |
| 7e | p-FC6H4 3f | 4f | 24 | 87 | 94 |
| 8 | m-CIC6H4 3g | 4g | 18 | 88 | 98 |
| 9f | p-CIC6H4 3h | 4h | 18 | 85 | 96 |
| 10f | 2-Furyl 3i | 4i | 18 | 83 | 96 |
| 11f | i-Pr 3j | 4j | 24 | 56 | 97 |
| 12 | n-Bu 3k | 4k | 24 | 50 | 97 |
| 13g | Cyclohexyl 3l | 4l | 24 | 49 | 91 |
| 14h | Et 3m | 4m | 24 | 52 | 96 |
| 15 | cyclopropane 3n | 4n | 24 | 52 | >99 |
| 16h | cyclopropane 3n | 4n | 24 | 77 | >99 |
| 17 | E-BDMSCH=CH 3o | 4o | 24 | 86 | 90 |
| 18i | PhCHCH 3p | 4p | 18 | 50 | 94 |
All reactions were conducted using 0.88 mmol both ethyl dlazoacetate 2 and the respective aldehyde 3a–p at a concentration of 1.0 M in THF unless otherwise noted.
Isolated yields.
Determined by chiral HPLC.
Reaction conducted on 1.76 mmol scale of benzaldehyde 3a using (R,R)-1.
Ethyl diazoacetate 2 added at ~12.5 uL/hr.
Reactions conducted at 0.5 M.
Reaction conducted using 10 mol % (R,R)-1, 20 mol % Bu2Mg, 10 mol % 17 and 2 equiv. of diazoester 2.
Reactions conducted using 2 equiv aldehyde with respect to ethyl diazoacetate 2.
See discussion.
