Table 8.
Diastereoselective Addition of Carbon Nucleophiles to β-Hydroxy-α-ketoesters A.
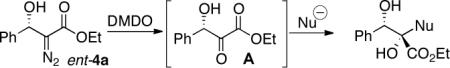
| Entrya | Conditions | Product | % yieldb | drc | ct (%)d |
|---|---|---|---|---|---|
| 1 |
|
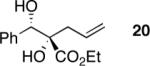
|
99 | >20:1e | 96 |
| 2 |
|
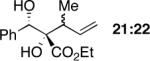
|
72 | >20:1 (1:1.3f) | 99,95 |
| 3 |
|
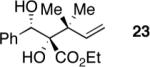
|
62 | >20:1 | >99 |
| 4 |
|
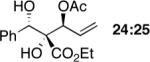
|
85 | >20:1e (5:1f) | 97,95 |
| 5 |
|
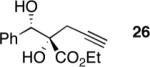
|
60g | >20:1e | >99 |
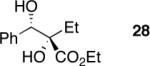
| 6 | Me2Zn |
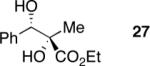
|
71 | >20:1h | 99 |
| 7 | Et2Zn |
|
80 | >20:1 | >99 |
| 8 |
|
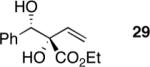
|
69 | 8:1 | 88 |
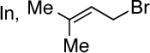
Conditions: (i) DMDO, concentrate; (ii) In (1.1 equiv), allyl halide (2.0 equiv) (entries 1–5); Me2Zn/Et2Zn/vinylzinc bromide (entries 6–8).
Combined isolated yield (over two steps).
Determined by crude NMR.
ct (%) (chirality transfer)=ee (%) product/ee (%) (S)-4a (HPLC).
Assigned by nOe on acetonide.
Diastereoselectivity at allylic position.
Isolated as a 5:1 mixture of homopropargyl alcohol and allenyl alcohol.
See supporting information for assignment.
