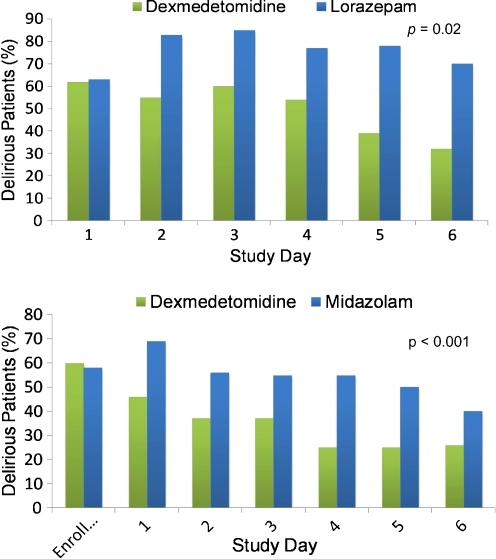
Fig. 1.
(a) A priori subgroup analysis of the Maximizing Efficacy of Targeted Sedation and Reducing Neurological Dysfunction (MENDS) randomized controlled trial. Compared to sedation with lorazepam, sedation with dexmedetomidine resulted in a markedly reduced daily risk of delirium (p = 0.02). As noted in this article, this relationship was even more pronounced in patients with severe sepsis. Note that both groups start with 60% prevalence of delirium in keeping with most cohorts of mechanically ventilated patients, and then there is a large difference in delirium prevalence throughout the remainder of the study period. Adapted from Pandharipande, et al. [132]. (b) In the Safety and Efficacy of Dexmedetomidine Compared with Midazolam (SEDCOM) trial comparing dexmedetomidine to midazolam for sedation of mechanically ventilated patients, the prevalence of delirium in dexmedetomidine-treated patients was significantly less than midazolam-treated patients (54% vs 76.6%; p < 0.001). As in (a), both groups started with 60% prevalence of delirium and then showed a large difference in delirium prevalence throughout next week. These (a) and (b) pose the unanswered question as to whether or not it is the avoidance of a gamma-amino butyric acid (GABA)-agonist or the receipt of an alpha-2 agonist that resulted in such marked delirium reduction. Adapted from Riker, et al. [55]