Abstract
Thymocytes must complete an elaborate developmental program in the thymus to ultimately generate T cells that express functional but neither harmful nor useless TCRs. Each developmental step coincides with dynamic relocation of the thymocytes between anatomically discrete thymic microenvironments, suggesting that thymocytes’ migration is tightly regulated by their developmental status. Chemokines produced by thymic stromal cells and chemokine receptors on the thymocytes play an indispensable role in guiding developing thymocytes into the different microenvironments. In addition to long-range migration, chemokines increase the thymocytes’ motility, enhancing their interaction with stromal cells. During the past several years, much progress has been made to determine the various signals that guide thymocytes on their journey within the thymus. In this review, we summarize the progress in identifying chemokines and other chemoattractant signals that direct intrathymic migration. Furthermore, we discuss the recent advances of two-photon microscopy in determining dynamic motility and interaction behavior of thymocytes within distinct compartments to provide a better understanding of the relationship between thymocyte motility and development.
Keywords: Thymocytes, Thymus, Chemokines, Migration, Development, Two-photon microscopy
Introduction
Unlike most other hematopoietic lineage cells that develop in the bone marrow, T lymphocytes develop in the thymus. Thus, a critical role of the thymus is to provide an environment to develop and select mature thymocytes that contain reactivity to a variety of foreign antigens but not to self-antigens. For example, αβ T cells undergo random rearrangement of their T cell receptor (TCR) α and β chain genes as they develop in the thymus, resulting in a potential repertoire of more than 1010 antigen specificities [1]. Among those cells, the thymus must foster the development of thymocytes with TCR specificities that provide immunity against a plethora of foreign pathogens, but at the same time eliminate thymocytes that express TCRs that are useless, or that might initiate attack to their own tissues. Thus, it is not surprising that thymocytes must pass through multiple checkpoints to ensure that only useful but not harmful T cells are released into the circulation and periphery.
The thymus plays a central and unique role in the adaptive immune system by providing an inductive environment to thymocytes during their development. It is composed of two lobes and within each lobe, histologically and functionally discrete regions exist. The adult thymus can be divided into a central medulla and a peripheral cortex enclosed by an outer capsule [2, 3]. Cells in the thymus are broadly divided into two categories; cells derived from hematopoietic stem cells that originate from the bone marrow, and resident stromal cells that are derived from non-hematopoietic lineage. Cells from the hematopoietic lineage in the thymus include T lymphocytes (thymocytes), dendritic cells, and very small populations of B cells, macrophages, and natural killer cells. Non-hematopoietic cells in the thymus include epithelial cells that reside either in the medulla or the cortex, mesenchymal cells, and endothelial cells. Thymic cortex and medulla are composed of distinct sets of cells from the hematopoietic and non-hematopoietic lineages. For instance, the cortex is composed of densely packed immature thymocytes supported by a network of cortical thymic epithelial cells (cTECs) and dendritic cells, whereas the medulla contains relatively fewer and loosely packed mature thymocytes supported by medullary thymic epithelial cells (mTECs) and dendritic cells. The cortex of the thymus is supplied by extensive networks of capillaries that are connected to postcapillary venules and arterioles at the corticomedullary junction (CMJ) [4].
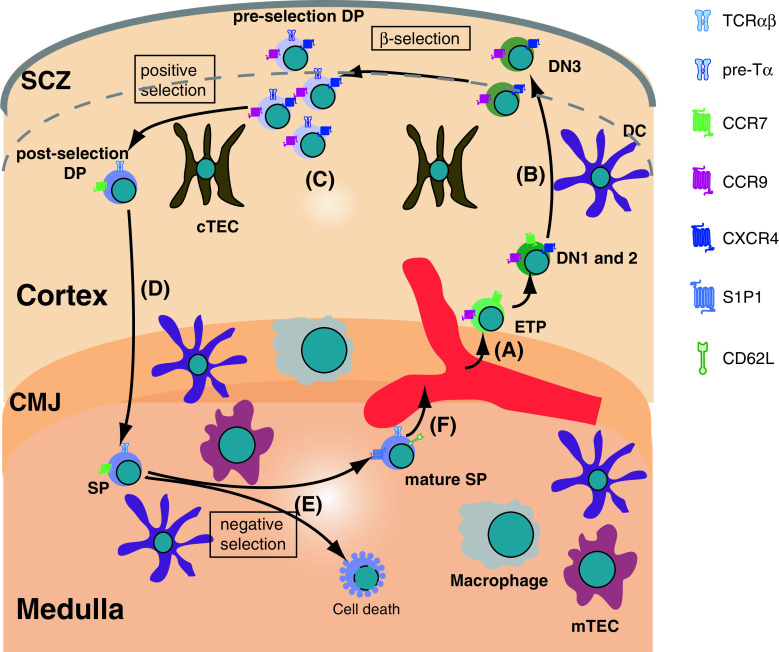
During their lifetime, thymocytes are in constant motion. Originally developing in the bone marrow, early progenitor cells circulate in the blood until they enter the thymus through blood vessels located at the CMJ. Inside the thymus, thymocytes transmigrate between anatomically distinct microenvironments where they are continuously interacting with resident stromal cells. These interactions are pivotal to accomplish maturation, proliferation, and selection of thymocytes. Thus, motility is one of the most fundamental characteristics of thymocytes. Development of T lymphocytes in the thymus correlates with their dynamic relocation between different microenvironments. Immunofluorescence studies using fixed tissues displayed a remarkable compartmentalization of thymocytes within the thymus, depending on their developmental status [2, 5]. For example, early thymic progenitors (ETPs) enter from the blood at the CMJ, commit to T cell lineage, and migrate outwardly towards the sub-capsular zone (SCZ) (Fig. 1a, b). During this migration, ETPs undergo subsequent developmental steps in the CD4- CD8- double-negative (DN) stage, ultimately reaching the SCZ. The DN cells advance to the CD4+ CD8+ double-positive (DP) cells as they are meandering through the cortex (Fig. 1c). The DP thymocytes undergo a critical developmental checkpoint called positive selection in the cortex to select the thymocytes that express a functional TCR. Thymocytes that successfully complete positive selection travel to the medulla and become CD4+ or CD8+ single-positive (SP) thymocytes (Fig. 1d). In the medulla, they undertake yet another critical developmental task called negative selection to eliminate thymocytes that are self-reactive (Fig. 1e). Finally, mature thymocytes in the medulla egress from the thymus to the blood stream to migrate to the peripheral lymphoid organs (Fig. 1f).
Fig. 1.
Overview of thymocyte migration. a The thymus settling progenitors (TSPs) enter the parenchyma of the organ near the cortico-medullary junction (CMJ) and differentiate into early thymic progenitors (ETPs), CD4- CD8- double-negative (DN)1 and DN2 not far away from the entry site. b DN3 thymocytes migrate to the sub-capsular zone (SCZ) attracted mainly by CXCL12 and CCL25, the ligands for CXCR4 and CCR9, respectively. Near the SCZ, thymocytes undergo β-selection and the cells that successfully pass this checkpoint proliferate extensively to give rise to CD4+ CD8+ double-positive (DP) thymocytes. c DP thymocytes migrate within the cortex in search for the rare selecting peptide-MHC molecules presented on the cortical thymic epithelial cells (cTECs). d Cells that successfully undergo the positive selection checkpoint up-regulate the chemokine receptor CCR7 and migrate to the medulla. e In the medulla, most of the auto-reactive cells undergo negative selection, while the rest of the SP thymocytes undergo maturation. f The mature self-tolerant SP thymocytes emigrate into the circulation at the large blood vessels at the CMJ. The most important receptors that guide the migration of thymocytes are depicted, as well as markers of different developmental stages and important stromal cell types
The differential localization of thymocytes at different developmental stages suggests that there is concerted regulation of thymocyte migration and motility at each step of their maturation. Attraction, repulsion, and adhesion are the major processes underlying migration. Major providers of attractive (and possibly repulsive) signals in the thymic microenvironment are the chemokines. Chemokines are a group of low-molecular-weight, mostly basic, structurally related molecules that drive migration of cells through interactions with a subset of seven-transmembrane, G protein-coupled receptors [6, 7]. Chemokines function as guidance cues, either as soluble gradients or as anchored forms on the extracellular matrix [8]. Furthermore, chemokines also increase a cell’s intrinsic motility in a non-directional, random manner [9, 10]. Thymic stromal cells as well as dendritic cells are rich sources of chemokines (Table 1). Interestingly, chemokines exhibit differential expression patterns in the distinct thymic microenvironments, suggesting that they play a role in selectively recruiting specific thymocyte populations. Thymocytes at different stages of development express different chemokine receptors allowing them to relocate between distinct microenvironments that express the corresponding chemokines (Table 1) [6].
Table 1.
List of chemokines and their receptors that are expressed in the thymus
| Chemokine | Chemokine-expressing cells | Receptor | Receptor-expressing cells in the thymus |
|---|---|---|---|
| CCL25 (TECK) | Thymic primordium [22] | CCR9 | Bone marrow-derived T-lineage precursors and ETPs [11, 23] |
| cTECs and mTECs [40, 41] | DN3-DN4, DP and a subset of SP thymocytes [45] | ||
| Thymic dendritic cells and endothelial cells [42] | DP thymocytes and CD62L− CD69+ SP thymocytes [40, 43, 46, 137, 138] | ||
| CXCL12 (SDF1-α) | cTECs [33] cTECs and | CXCR4 | DN1 to DN4 thymocytes [33, 37, 139] |
| mTECs [41] | DN, DP, and a subset of SP thymocytes [37, 139 ] | ||
| Blood venules in the cortex and CMJ [34, 59] | |||
| CCL19 (ELC, MIP3β) | mTECs [41, 75, 80], endothelial venules in the medulla [41, 80, 121] | CCR7 | DN1 and DN2 thymocytes [41] SP thymocytes [41, 75, 80, 81, 137, 139] |
| CCL21 (SLC) | Parathyroid primordium [22] mTECs [41, 75, 121] | TCR-stimulated DP thymocytes [75] | |
| CCL17 (TARC) | Dendritic cells [140, 141] | CCR4 | CD3 + CD4 + CD8low thymocytes [81] |
| CCL22 (MDC) | A subset of mTECs, outer walls of Hassall’s corpuscles [80, 81] | CD62L−CD69+ SP thymocytes [137] | |
| CD4 + CD8low and DP thymocytes [80] |
The synonyms for the chemokine are shown in parentheses
CCL CC-chemokine ligand, CCR CC-chemokine receptor, CXCR CXC-chemokine receptor, CXCL CXC-chemokine ligand, SDF stromal-cell-derived factor, TECK thymus-expressed chemokine, ELC Epstein–Barr virus-induced molecule 1 ligand chemokine, MIP3β macrophage inflammatory protein 3β, SLC secondary lymphoid-tissue chemokine, MDC macrophage-derived chemokine, TARC thymus and activation-regulated chemokine, CMJ cortico-medullary junction, cTECs cortical thymic epithelial cells, mTECs medullary thymic epithelial cells, DN double negative, DP double positive, SP single positive, TCR T-cell receptor
In addition to chemokines, other less well-studied guidance molecules in the thymus include Eph kinases, ephrins, semaphorins, plexins, and neuropilins. Integrins, on the other hand, are pivotal for adhesion to the extracellular matrix or other surface molecules. Eph kinases are a large family of receptor tyrosine kinases whose ligands are the ephrins. They can regulate the cytoskeleton and have well-described roles in the development of the neural system (e.g., axon guidance), angiogenesis, bone, and intestinal and glucose homeostasis (reviewed in [11]). Most of the family members are expressed in the thymus on stromal cells as well as on thymocytes [12, 13]. Their roles in axon guidance and expression in the thymus have led to hypotheses that Eph kinases and/or ephrins may also play a role as guidance cues in the thymus. Indeed, interference with Eph/ephrin signaling with recombinant protein stimulation in thymic organ cultures has shown quite dramatic effects on T cell development [14–16]. However, it is unclear exactly what the mechanism is underlying the effect of the treatments. In addition, mice lacking several of the Eph kinases or ephrins (EphB6, EphA4, and ephrinB2) do not show cell-autonomous disturbances in the T cell development, although EphB2 is critically required [15, 17–19]. One possible explanation for the lack of an obvious phenotype in some of the individual knock-out mice is the high degree of promiscuity in the interactions between Eph kinases and ephrins. In addition, because these molecules transmit signals between stromal cells and thymocytes in both directions, it is hard to separate primary from secondary defects in the respective knock-out animals. For example, EphA4 and EphB2 are essential regulators of stromal cell development in the thymus [15, 20]. In their absence, the overall architecture of the thymus and the morphology of TECs are abnormal. However, the development of wild-type thymocytes on EphA4−/− or EphB2−/− thymus stroma is also defective because the stromal cells are unable to direct proper T cell differentiation. Finally, recent data suggest that ephrinB2 expression on neural crest-derived thymic mesenchymal cell is important for the migration of the thymus into the thoracic cavity during embryonic development [17]. In its absence, the thymus is retained in the cervical region. Quite interestingly, T cell development is unperturbed in this case.
Semaphorins, initially identified as repulsive axon guidance signals that direct neuronal axons to the right direction [21], have been implicated in thymocyte migration. Semaphorins are membrane-bound or secreted proteins that contain a conserved extracellular N-terminal “Sema” domain [22, 23]. They bind to at least two families of receptors, plexins and neuropilins (NPs). The plexins are large transmembrane glycoproteins that function as receptors for semaphorins directly or indirectly by binding to NPs, another group of transmembrane glycoprotein receptors that interact with semaphorins. Although their participation in the development of the nervous system (e.g., in axon guidance) is well known, what role semaphorins play in the immune system is incompletely understood [23–25]. Semaphorins expressed on immune cells are called “immune semaphorins” and are involved in regulating immune cell migration and T cell-APC interactions, thereby leading to mounting proper immune responses in the periphery [22, 23]. Likewise, some semaphorins and their receptors are expressed in the thymus and contribute to thymic development. For instance, secreted class III semaphorins, Sema 3A, 3B, 3C, and 3D, interact with NP-1 [22, 26]. In the human thymus, NP-1 is expressed at low levels on all CD4 and CD8 subsets and at high levels on microenvironment cells including TECs and DCs [26, 27]. Sema3A, a natural ligand for NP-1, is expressed in human cortical and medullary TECs as well as all subsets of thymocytes at high levels and regulates human thymocyte adhesion onto TEC layers [26, 27]. However, thymocyte development in NP-1-deficient mice is normal, suggesting NP-1/Sema 3A interaction can be replaced by other molecules [26]. Interestingly, Sema3E/Plexin D1 play an important role in proper positioning of positively selected thymocytes by regulating chemotactic responses. We will discuss this in more detail below.
Integrins are transmembrane heterodimers composed of one α and one β subunit. They serve as adhesion molecules and receptors for mechanical stimulation and bind to extracellular matrix proteins or cell-surface molecules [28]. Integrins are very important for immobilization of cells on two-dimensional surfaces, especially under shear stress conditions [29]. For example, they are essential for the extravasation of the various blood cells into the tissues. However, firm adhesion appears to play little role for the motility in three-dimensional matrix (e.g., the thymus parenchyma) outside the blood vessels, as the thymocytes are in constant motion [30]. Nevertheless, some initial data suggested that integrins might play a role during the T cell development. For example, transgenic expression of an inhibitor of integrin function in the thymus led to a partial block in the DN-DP transition and increased production of CD8+ T cells at the expense of CD4+ T cells [31]. However, none of the various integrin or their cell surface receptor (vascular cell adhesion molecule1 (VCAM-1) and intracellular adhesion molecule 1 (ICAM-1)) knock-outs have demonstrated a remarkable thymus phenotype [32–43]. A possible exception could be the process of negative selection (see below) that might involve cell–cell interactions reminiscent of the immunological synapse in which integrins (αLβ2) and their ligands (ICAM-1) are known to play important roles.
The studies of cellular localization using fixed tissues have provided a snapshot view of migration patterns of different populations of thymocytes as well as compartmentalization of epithelial cells within the thymus. Furthermore, it also provided critical information about expression patterns of chemokines in discrete thymic microenvironments. However, dynamics of thymocyte motility, migration, and interaction with residential cells within or between each microenvironment cannot be determined using this method. To visualize thymocyte motility and interaction within the intact thymic environment, immunologists have successfully adopted two-photon microscopy. In this review, we will summarize previous findings about thymocyte migration using fixed tissues, which were fundamental to formulate the current thymocyte migration model, and discuss recent advances in studying thymocyte motility, migration, and interaction using two-photon microscopy. Finally, we will provide discussions on what questions need to be further addressed to better understand the relationship between thymocyte motility and development.
Seeding of the thymus at the prevascular stage
Since the thymus does not contain self-renewing stem cells, a steady supply of progenitor cells originating from the bone marrow is inevitably required for continuous thymopoiesis [44–49]. Although some of the early progenitor cells in the bone marrow and blood display a potential to differentiate into thymocytes, the identity of thymus settling progenitor cells (TSPs) is not clear [50, 51]. Nevertheless, the most immature progenitor cells in the thymus with a potential to develop to thymocytes have been identified as the early thymic progenitors (ETPs) [51]. The bona fide ETPs are defined as the c-Kithi IL-7Rαneg/lo subset within the most immature DN subpopulation DN1 (CD25-CD44+) as only this subset contains a measurable T lineage differentiation potential [48, 52].
T lymphoid progenitor cells begin to colonize the thymic primordium generated by the third pharyngeal pouch endoderm at gestation week 7 to 8 in humans and at embryonic day 11.5 in mice, prior to development of vasculature [53, 54]. The chemokines CCL25 and CCL21 are expressed in the thymic primordium and neighboring parathyroid primordium and coordinate fetal thymus colonization before its vascularization [55]. Mice deficient for both of their counterpart receptors, CCR9 and CCR7, respectively, display substantial defects in fetal thymus colonization, resulting in a decrease to only 2% of wild-type (WT) thymocyte numbers in the E14.5 fetal thymus [55]. In a recent study, the role of CXCR4 in this process was examined [169]. Using mice that are deficient in either one, two or three of the chemokine receptors, CCR7, CCR9 and CXCR4, the authors found that thymopoiesis was most severely impaired in the CCR7/CCR9/CXCR4 triple-deficient thymic rudiments of E14.5 mice, even more so compared to the CCR7/CCR9 double-deficient mice. Together, these results indicate that combined chemokine receptor signals on the thymic progenitor cells in response to CCL25, CCL21 and possibly CXCL12, are central for fetal thymus colonization at the stage prior to development of vasculature.
Another molecule that has been suggested to play a role in the colonization of fetal thymus is EphB2 kinase [57]. What is remarkable about EphB2 is that its expression is required on both the stromal cell and the TSPs for efficient colonization to occur. The exact function of this molecule is unclear at the moment, however, it has been suggested that EphB2 can regulate chemokine expression and the deposition of extracellular matrix.
Recruitment of hematopoietic progenitors to the adult thymus
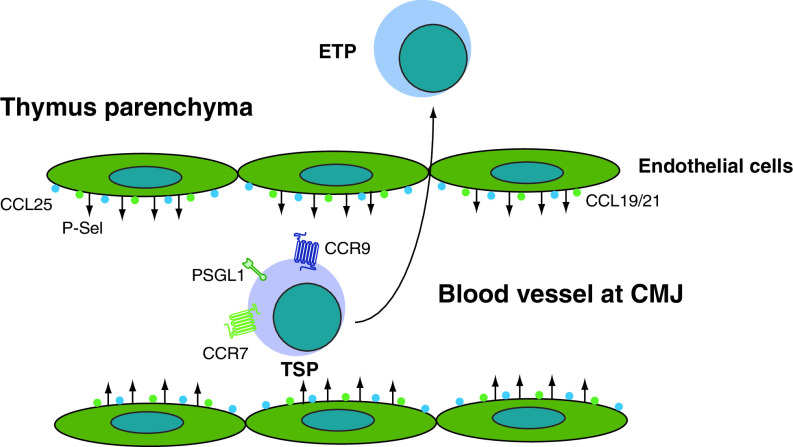
In the postnatal and adult thymus that contains vasculature, TSPs enter the thymus from blood via postcapillary venules, which are located at the CMJ (Fig. 1a). Thymus seeding occurs periodically alternating non-receptive and receptive stages, suggesting there might be feedback signals from the thymus to regulate recruitment of TSPs [49]. Although the identity of these cells and the thymus-specific signals that recruit them from the blood are incompletely understood, recent findings indicate that adhesion and chemokine signals may play an important role. For instance, P-selectin is expressed on thymic endothelial cells and its ligand PSGL-1 is expressed on the progenitor cells in the bone marrow, blood, and thymus (Fig. 2). Binding of P-selectin to thymic endothelium via PSGL-1 is required for efficient recruitment of TSPs, since PSGL-1 deficiency results in fewer numbers of ETPs [58]. Interestingly, expression levels of P-selectin together with CCL25 on thymic endothelial cells correlate with the occupancy of the intrathymic niche, suggesting they may function as a feedback loop or thymic gatekeepers [59].
Fig. 2.
Factors that regulate recruitment of hematopoietic progenitors to the thymus. The thymus settling progenitors (TSP) use CCR9, CCR7, and PSGL1 to recognize CCL25, CCL19/21, and P-selectin (P-sel) on the large blood vessels at the cortico-medullary junction and efficiently enter the thymic parenchyma
Two recent papers highlight the importance of chemokine signals for thymic settling capacity of hematopoietic progenitors to the adult thymus [44, 56]. Using CCR7 and CCR9 double-deficient mice, both papers showed that the loss of both receptors severely impaired the recruitment of TSPs, which resulted in a near 100-fold decrease in numbers of the ETPs. On the contrary, total thymic cellularity was not changed in CCR7 and CCR9 double-deficient mice, suggesting that a compensatory mechanism exists. Indeed, higher proliferation was found in the DN3 (CD44-CD25+) compartment of the CCR7 and CCR9 double-deficient mice. Moreover, the thymic environment of CCR7 and CCR9 double-deficient mice also supported the enhanced expansion of DN3 and DP thymocytes.
Based on the current paradigm of tissue homing, a role for integrins can be expected in the migration of BM progenitors to the thymus. Indeed, antibody-blocking experiments have suggested that α4β1, αLβ2 [lymphocyte function-associated antigen 1 (LFA-1)] as well as another adhesion molecule, CD44, might play a role in thymic homing [60, 61]. However, more thorough investigation of the subject is warranted, because none of the respective knock-out mice (α4-integrin−/−, αL-integrin−/−, β1-integrin−/−, β2-integrin−/−, CD44−/−) have been described to have a defect in T cell development in the thymus [32–35, 37–39, 62].
Outward migration of the DN cells; from the CMJ to the sub-capsular zone
Once recruited from the blood to the thymus, ETPs remain at the CMJ for an average of 10 days and undergo 1,000-fold expansion to become DN1 cells [2, 63]. The retention, expansion, and release signals at the CMJ are largely unknown. However, the c-Kit ligand, IL-7, and Hedgehog signals have been suggested to participate in proliferation and differentiation of the DN1 cells [2, 64]. In addition, Notch ligand signal specifies T-lineage commitment [65–69]. Once DN1 cells start to increase expression of the RAG genes and CD25, they differentiate to the DN2 stage. The DN2 cells undergo their maturation as they migrate outwardly from the inner cortex to the outer cortex, finally reaching the sub-capsular zone (SCZ) as DN3 thymocytes (Fig. 1b). The β-selection, a critical checkpoint that screens for the productive rearrangement of the TCRβ chain and assembly with the pre-TCRα chain, begins at the DN3 stage of thymocyte development. A functional pre-TCR complex generates survival, proliferation, and differentiation signals to ensure successful transition to the DN4 stage. Thymocytes that successfully rearrange their TCRβ differentiate to the DN4 then to the DP stage in the SCZ.
CXCL12, the ligand for CXCR4, is expressed on scattered cTECs [70], blood venules in the cortex and SCZ [71], and may guide the DN cells to migrate across the cortex to reach the SCZ (Fig. 1b). However, the role of CXCR4 in early thymocyte development has been controversial. Earlier studies using conventional CXCR4 knock-out (KO) mice revealed the role of CXCR4 in cardiac development, fetal cerebella development, B lymphopoiesis, and myelopoiesis, resulting in embryonic lethality [72, 73]. However, these studies showed that T lymphopoiesis was normal at E17.5-18.5 in CXCR4 KO embryos. Fetal thymic organ culture (FTOC) or engraftment of the fetal thymus under the kidney capsule of TCRα-deficient mice revealed that maturation to SP thymocytes and emigration to the periphery were normal in the absence of CXCR4. In contrast, other groups reported impaired thymopoiesis including substantial decrease in DP thymocyte numbers at E17.5 and 18.5 in CXCR4-deficient embryos [74, 170]. The discrepancy between the results of the aforementioned studies is difficult to explain at present. Moreover, CXCR4-deficient thymic precursors showed a substantial disadvantage over WT counterparts in reconstituting irradiated adult recipients [70, 74–76]. For example, competitive reconstitution experiments using CXCR4-deficient fetal liver cells together with WT bone marrow cells showed severe disadvantage of CXCR4-deficient precursor cells to repopulate DN1, DN2, DP, and mature CD4 SP compartments [74]. In hematopoietic chimeras where CXCR4 is conditionally deleted using Lck-Cre mouse line that express Cre recombinase at DN1 stage under control of the proximal Lck promoter, CXCR4-deficient cells were arrested at the DN1 stage and accumulated at the CMJ due to their failure to migrate away from the CMJ [70]. This result indicates that CXCR4 signals at the DN1 are critical for initiating the outward migration at the CMJ and subsequent differentiation under competing conditions [70]. A recent study demonstrated that deleting CXCR4 at the DN2 and DN3 stage using a Cre recombinase under the control of the Lck promoter (that is distinct from the one used in the previous study) leads to marked inhibition in the transition from the DN3 to DN4 stage as well as from the DN4 to the DP stage, resulting in thymic atrophy, accumulation of DN3 cells, and reduced numbers of DP cells [71]. Furthermore, CXCR4-deficient CD25+ DN cells (DN2 and DN3 cells) accumulated in the lower inner cortex area rather than at the SCZ where WT counterparts were recruited, suggesting that CXCR4 is involved in polarized migration of the DN2 and DN3 cells to the SCZ [71, 170]. Intriguingly, CXCR4 also facilitates β-selection by providing optimal pre-TCR-induced proliferation and survival signals, suggesting a role of CXCR4 as pre-TCR co-stimulator.
Another chemokine that coordinates directed migration at the DN3 stage is CCL25 (TECK), the ligand of CCR9. There are conflicting data about the expression of CCL25, but most likely it can be found in both cortical and medullary TECs as well as medullary dendritic cells [77–79]. Expression of CCR9 is highly regulated at multiple stages in thymocytes, suggesting a specific role of CCL25-mediated migration in the development of thymocytes. For example, expression of CCR9 begins at the DN3 stage and dramatically increases following pre-TCR stimulation or after the DN4 to DP transition, and then decreases at the mature CD4 SP thymocyte stage [80, 81]. Interestingly, CCR9-deficient DN3 cells failed to accumulate at the SCZ and displayed scattered distribution pattern [82]. However, thymic development in the absence of CCR9 was found to be normal, suggesting aberrant localization of CCR9- deficient DN3 thymocytes did not compromise thymic development. Developmental defects of CCR9 deficiency were only revealed either under competing or overexpression conditions. For instance, CCR9- deficient thymocytes were out-competed by WT thymocytes at multiple stages of T cell development. Overexpression of CCR9 at the DN3 stage using CCR9- transgenic mice resulted in a substantial reduction in DP thymocyte numbers, indicating that perturbed migration at the early stages of thymocyte development inhibited optimal differentiation [83].
Thymocytes that successfully undergo β-selection at the DN3 stage down-regulate CD25 expression and increase expression of CD4 and CD8 co-receptors to become DP thymocytes. In contrast to the outward directional migration of the DN thymocytes, DP thymocytes relocate away from the SCZ and randomly migrate throughout the cortex (Fig. 1c). The cues that turn off the retention signal at the SCZ and promote random migration in the cortex are not known.
Thymic crosstalk between thymocytes and stromal cells in the cortex
Thymocytes constantly interact with thymic epithelial cells in the cortex and medulla and this interaction is required to complete their development. In parallel, cortical and medullary epithelial cells also require the presence of thymocytes for their differentiation [84–86]. Thymocytes from human CD3ε transgene homozygous mice are blocked at a very early stage of development with only 2–3% of thymocytes progressing to the DN1 stage. Interestingly, the thymus from these mice is abnormal. For instance, it is 1% of normal thymus size and its architecture is completely disorganized. Further characterization revealed that their epithelial cells are arrested at an immature stage, unable to differentiate to mature cortical epithelial cells [86]. In contrast, the thymus from Rag1−/− or Rag2−/− mice, in which thymocyte development is arrested at the DN3 stage, contains cTECs and histologically normal cortex, but their medulla is small [84–86]. These results suggest that the differentiation of thymocytes from the DN1 to the DN3 stage controls the maturation of cTECs and formation of cortex in the thymus.
Migration of DP thymocytes within the cortex
Within the cortex, DP thymocytes must undergo a developmental task called positive selection. They rearrange their TCRα gene locus to generate TCRα chain that pairs with TCRβ chain and finally form a mature TCR. DP thymocytes with the newly formed TCR search for a peptide-MHC (pMHC) complex on the cortical epithelial cells, which can interact with their TCR with moderate affinity and avidity. This TCR-pMHC interaction provides signals required for further differentiation of DP thymocytes into mature SP thymocytes. Small numbers of cells are positively selected and differentiate into CD4 or CD8 SP thymocytes. Failure of such interaction results in death by neglect of DP thymocytes. Following positive selection, mature CD4 or CD8 SP thymocytes increase expression of chemokine receptors such as CCR7, which allows them to migrate from the cortex to medulla, where the ligands of CCR7 are expressed (Fig. 1d).
Studying thymocyte motility in the cortex using two-photon microscopy
Historically, localization of thymocytes during their development has been studied in static settings using methods such as immunohistochemistry or immunofluorescence in fixed tissue sections. Advances in two-photon laser-scanning microscopic imaging have made it possible to visualize dynamic migration and interaction of thymocytes in fetal or reaggregate thymic organ cultures (FTOC or RTOC), thymic slices, and explanted thymi [87–91]. Two-photon microscopy has proven to be a powerful tool to investigate spatial and temporal migratory behaviors of thymocytes in real time in organ cultures or intact tissues. Some of the initial experiments using two-photon microscopy described the patterns of thymocyte motility during positive selection [92]. In these studies, RTOC were generated with thymocytes bearing a fixed TCR co-cultured with a mixture of thymic stromal cells, which were composed of unlabeled MHC-deficient stromal cells mixed with fluorescently labeled MHC-sufficient WT thymic stromal cells. Strikingly, thymocytes were highly motile, actively crawling on the surface of stromal cells with range of mean velocities from 6 to 15 μm/min. Under the conditions that support positive selection, MHC recognition increases the duration of the thymocyte-stromal cell interaction in both stable and dynamic interactions. Although this study shed light on thymocyte motility in a three-dimensional organ culture, the lack of normal organ architecture makes it difficult to study the long-range migration of thymocytes between distinct regions of the thymus at different developmental stages and to determine motility within a native microenvironment.
To elucidate motility and long-range migration of thymocytes inside of the intact cortex, experiments to visualize thymocyte motility within intact thymic lobes using two-photon microscopy were performed [93]. Partial bone marrow chimeras were generated, in which only 1% of total thymocytes were fluorescently labeled to facilitate tracking of individual cells. From this study, cortical DP thymocytes before positive selection showed random walk migration with relatively low motility rates between 3 and 8 μm/min. Surprisingly, there was also a small number of thymocytes that exhibit fast and directed migration to the medulla with motility rates of 10 μm/min or greater. The appearance of this population was correlated with positive selection, implying that these thymocytes may represent positively selected cells that are on their way to the medulla. Expression of CCR7 is increased following positive selection and ligands of CCR7 are expressed preferentially in the medulla, suggesting that post-positive selection thymocytes are selectively recruited to the medulla via CCR7 [94]. Thus, authors of this paper concluded that positive selection leads to a rapid directional migration pattern, increased proportion of fast and directionally migrating cells as well as increase in overall migration rate, which was not observed in the earlier studies using RTOC.
The ability to visualize thymocyte movement in a three-dimensional microenvironment using two-photon microscopy opened many avenues to address important questions regarding thymocyte migration and signaling within normal tissue environment during development. For example, to determine the effect of calcium signals on thymocyte motility during positive selection, Bhakta et al. monitored the motility of calcium indicator-labeled thymocytes in a thymic slice using two-photon microscopy [1]. The thymic slice technique offers several advantages over RTOC or FTOC. Unlike RTOC or FTOC that require many hours to form a three-dimensional structure, thymic slice preparation enables the acute introduction of labeled thymocytes of known subsets into intact thymic environment. In addition, the native architecture of the medulla and the cortex is preserved and residential cells in these areas are conserved, thus generating an ideal model system to study thymic development and the long-range migration that accompanies it. Using this system, the authors of this paper found that naive thymocytes are motile at low intracellular calcium concentrations with a mean motility rate of 3.5 μm/min, but become immotile with sustained calcium concentration oscillations during positive selection. Further, they showed that an increase in intracellular calcium concentration is necessary and sufficient to arrest thymocyte motility. From these results, the authors speculated that sustained calcium flux during positive selection prolongs the interaction with pMHC-bearing stromal cells and may facilitate gene transcription.
The cortical thymic stromal cells contain not only epithelial cells but also dendritic cells (DCs). DCs in the cortex may provide a functionally distinct microenvironment within the cortex. The interaction between thymocytes and DCs in the cortex during positive selection has been studied using two-photon microscopy [95]. This study revealed that dendritic cells localize near blood capillaries and CCL21 positive areas in the cortex. CCL21, one of the ligands of CCR7, is expressed at high levels in the medulla and low levels in the cortex. This unique vasculature-associated cortical microenvironment attracts DCs and thymocytes that are receiving positive selection signals in a CCR7-dependent manner. For instance, CCR7 transgenic thymocytes exhibited increased frequency and duration of the DC-thymocyte association with a slightly decreased average migration speed. CCR7-deficient thymocytes with a TCR transgene showed decreased interaction with DCs and increased motility. These results demonstrate that a specific microenvironment that contains chemokines can affect interaction of thymocytes with residential cells as well as their motility.
Role of GIT2 in thymocyte migration and positive selection
Although thymocyte migration within the cortex is thought to be important for positive selection and further development, whether defects in thymocyte migration indeed affect positive selection is unclear. Moreover, the molecular mechanisms of regulating chemokine-mediated chemokinesis as well as chemotaxis of the DP thymocytes are not well understood. Recently, we described that impaired motility of DP thymocytes does influence positive selection in GIT2−/− mice [96]. The GIT family members are multi-functional proteins characterized by the N-terminal GTPase activating protein (GAP) domain for ADP-ribosylation factor (Arf) family of small GTP-binding proteins [97–101]. In thymocytes and peripheral T cells, GIT2 is the predominantly expressed isoform. GIT1 and GIT2 display Arf GAP catalytic activity towards Arf1 and Arf6 [102, 103], which influence membrane traffic and actin remodeling [99].
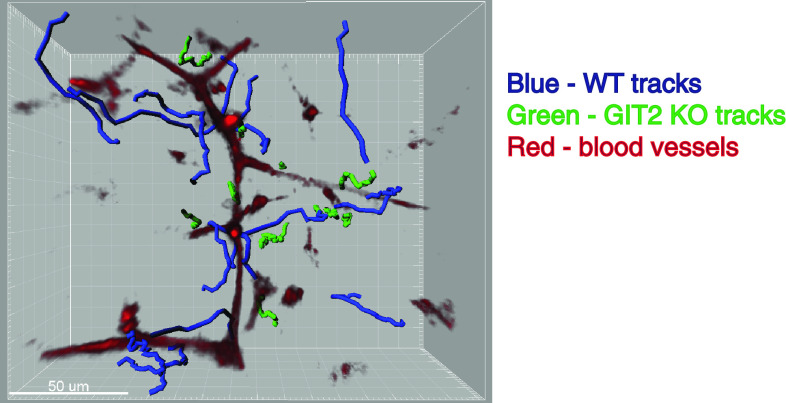
GIT2 regulates thymocyte motility in response to several chemokines. We found that migration to chemokines such as CXCL12 and CCL25 by GIT2−/− DP thymocytes was increased using a transwell migration assay. Moreover, GIT2−/− thymocytes exhibited exaggerated Rac activation and actin polymerization, and responded more to CXCL12 in a collagen matrix. Intriguingly, analysis of thymic development in GIT2−/− mice revealed that the generation of mature CD4 SP thymocytes in TCR transgenic GIT2−/− mice was severely impaired due to inefficient positive selection. However, the TCR signaling ability as well as cell death and apoptosis of GIT2−/− thymocytes were normal. We set forth to determine whether GIT2 deficiency affects thymocyte motility in vivo using two-photon laser scanning microscopy to monitor the migratory behavior in intact thymic lobes. We found that, in contrast to control DP thymocytes that migrate vigorously in the cortex and scan the cortical areas, GIT2−/− thymocytes accumulated in certain areas of the cortex including adjacent to CXCL12-positive small blood vessels with decreased motility (Fig. 3). As a result, the scanning activity of GIT2−/− thymocytes was severely compromised. We proposed that the in vivo migratory defect of the GIT2−/− thymocytes was a steady-state consequence of thymocytes that were trapped by an exaggerated response to local chemokine gradients in the cortex. Consequently, the limitation of GIT2−/− thymocytes to migrate and scan the cortical area ultimately compromised their ability to receive proper TCR signals for efficient positive selection.
Fig. 3.
Representative migratory tracks of wild-type and GIT2−/− thymocytes in the cortex. Thymi from a partial mixed bone marrow chimera were explanted and imaged by two-photon microscopy. Blood vessels are shown in red. Trajectories of individual cells, in blue (WT thymocytes) and green (GIT2−/− thymocytes), are shown as tracks. Note that GIT2−/− cells display much shorter tracks, reflecting their slow migration and minimal displacement. GIT2−/− cells are often found in areas around small blood vessels in the cortex where expression of chemokines is also detected
Migration from the cortex to the medulla
The medulla represents a unique environment for inducing tolerance and screening against the self-antigens presented there to prevent autoimmunity. Thus, migration from the cortex to the medulla is essential for the elimination of at least some of the self-reactive thymocytes. For example, mice with defective medulla development (RelB−/−, TRAF6−/−, NF-κB2−/−), mice that express MHC II only in the cortical but not in the medullary TECs (K14-MHC II on MHC II−/− background), or mice with defects in attracting positively selected DP thymocytes to medullary chemokines (CCR7−/− or plt/plt mice) all develop autoimmune disorders [94, 104–107].
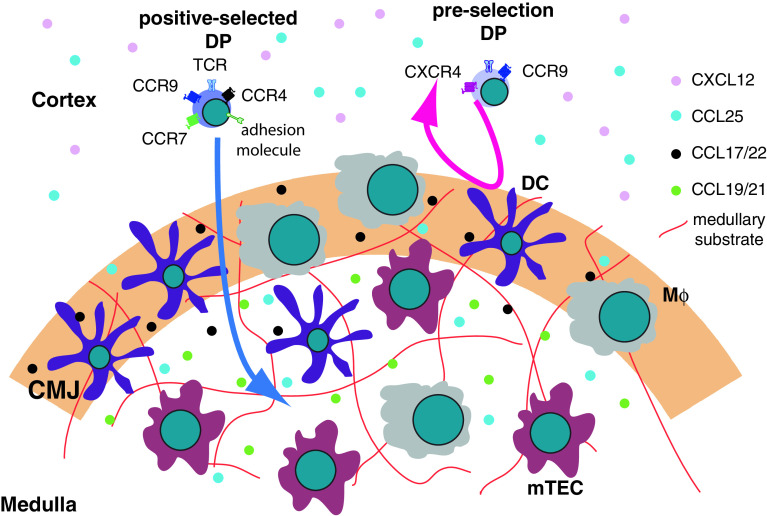
The most important factor guiding the migration of positively selected TCRαβ thymocytes into the medulla is signaling from the chemokine receptor CCR7 (Fig. 4). The ligands for CCR7 are CCL19 and CCL21. Their expression is much higher in the medulla than in the cortex and this gradient is thought to direct the positively selected DP thymocytes into the medulla [78, 94]. CCR7 expression starts after the positive selection [94]. A significant proportion of CD69hi TCRβhi DP (post-positive selection) and the majority of SP thymocytes express CCR7 on their surface. More MHC I-restricted than MHC II-restricted DP cells express CCR7 [108]. This fact can potentially provide a mechanistic explanation for the kinetic signaling model for CD4 vs. CD8 lineage commitment [109]. According to this model, CD4SP cells are selected by a strong and prolonged interaction with the cTECs, while CD8SP cells are selected by weaker and shorter signals. Perhaps the earlier expression of CCR7 on CD8SP-committed progenitors instructs them to up-regulate their speed and migrate towards the medulla, thus shortening the interaction time with the selecting TECs and decreasing the intensity of the TCR signal.
Fig. 4.
Migration from the cortex to the medulla. Post-selection DP thymocytes up-regulate CCR7 and migrate towards the medulla. CCR4 and CCR9 may also participate in this process. Another critical element for successful crossing the CMJ is the expression of an adhesion molecule that enables the selected thymocytes to crawl on the medullary substrate. The molecular identity of the adhesion molecule or medullary substrate is currently unknown. Pre-selection DP thymocytes do not express CCR7 and cannot migrate on the medullary substrate. mTEC medullary thymic epithelial cells, DC dendritic cells, Mϕ macrophages
The first clues for the participation of chemokine receptors in the migration across the CMJ came from experiments showing that this process could be inhibited by pertussis toxin (PTx) [110]. PTx is a well-known inhibitor of G-protein coupled receptors (GPCRs) that signal through Gαi. Most chemokine receptors fall in this category. The critical roles of CCR7 and its ligands in the migration towards the medulla were uncovered by studying the phenotype of gain-of-function and loss-of-function mouse mutant strains. In mice ectopically expressing CCR7 on the majority of thymocytes, DP cells gained the ability to cross the CMJ and invaded the medulla [111]. On the other hand, in CCR7-deficient mice or in plt/plt mice that lack expression of both ligands of CCR7, SP cells failed to migrate to the medulla and accumulated in the cortex [112]. These experiments suggested that CCR7 might be both necessary and sufficient for migration to the medulla.
Recently, the roles of CCR7 and its ligands for the cortex-to-medulla migration were examined in real time by Ritchie-Ehrilch et al. [113]. Using thymic slices overlaid with purified thymocyte populations, they described three distinct effects of CCR7. First, CCR7−/− CD4SP thymocytes were considerably slower than their wild-type (WT) counterparts, suggesting that CCR7 ligands have a strong chemokinetic effect. Second, unlike WT cells, CCR7−/− CD4SP did not display a directional bias towards the medulla, arguing that CCR7 ligands have a pronounced chemotactic effect as well. These results were also recapitulated with WT CD4SP cells treated with PTx, suggesting that CCR7 is the major chemokine receptor responsible for the increased speed of CD4SP thymocytes and their migration towards the medulla. Third, and most surprisingly, CCR7−/− thymocytes were found in the medulla in large numbers. At first glance, this finding appears at odds with previous data that CCR7 is necessary and sufficient for migration into the medulla [111]. However, CCR7−/− mice were shown to harbor a significant, albeit reduced, number of SP thymocytes in the medulla [114], implying that CCR7 might not be required for crossing the CMJ. Treatment with PTx prevented WT CD4SP from entering the medulla, suggesting that another chemokine is responsible for the entrance. The identity of this chemokine is unknown at present.
Other chemokines that could participate in the cortico-medullary migration of positively selected DP thymocytes are CCR9 and CCR4 (Fig. 4). CCR9 is expressed on almost all DP and CD8SP and on semi-mature CD4SP [115], and its ligand CCL25 can be found in both the cortex and the medulla [77, 78]. According to one report, although pre-selection and post-selection DP thymocytes express similar levels of CCR9, post-selection DP thymocytes respond to CCL25 more efficiently [116]. CCR4 is expressed on a small proportion of DPs (most likely post-positive selection) and on the semi-mature CD4SP [110], but not on CD8SP. The ligands for CCR4, CCL17, and CCL22, are expressed mostly in the medulla, especially on dendritic cells [117, 118]. That makes the ligands for CCR4 very good candidates to attract positively selected thymocytes. However, the development of thymocyte is not perturbed in the absence of CCR4 [119]. Nevertheless, thorough investigation of the dynamics of thymocyte migration is needed to determine the role of CCR4 in the cortico-medullary migration. Both CCL25 and CCR9 knock-out mice have been generated, but neither has shown any defect in the generation and localization of SP thymocytes [82, 120, 121]. These data suggest that both CCR9 and CCR4 may play redundant roles in directing migration across the CMJ. It is possible that these chemokine receptors play a redundant role with CCR7, or are responsible for the ability of SP thymocytes to crawl on a medullary substrate (see below).
Another important factor for the cortex-to-medulla migration is adhesion to the stromal substrate (Fig. 4). Although adhesion molecules are indispensable for certain types of motility, it was recently demonstrated that integrins on leukocytes are not required for migration on three-dimensional substrates [30]. In support of these data, the speed of SP thymocytes is often in the range of 15–20 μm/min—too fast for any kind of adhesion to form and dissolve [122]. However, it was recently reported that medullary substrate is the limiting factor for translocation from the cortex to the medulla [113]. The authors of this study reached this conclusion by trying to explain how the boundary between cortex and medulla forms. The sharp border suggested that it is unlikely that a diffusible attractant or repellent is present. They also excluded the presence of a specialized cellular barrier that prevents entry into the medulla. Instead, the authors proposed that a medullary substrate on which only SP thymocytes can crawl, or a surface-bound medullary repellent for DP thymocytes, determines the boundary. The identity of the medullary substrate or repellent and the corresponding receptors on thymocytes remain to be identified.
In addition to chemokines and adhesion molecules, chemorepellent signals have been suggested as another contributing factor involved in migration from the cortex to the medulla. Recently, it was demonstrated that the sema3E-plexinD1 signaling axis is important for the proper localization of CD69+ post-positive selection thymocytes to the medulla [116]. Expression of plexinD1 was up-regulated on thymocytes after positive selection, while sema3E was expressed mostly in the medullary stromal cells. Although sema3E had little potency to attract or repel thymocytes, it significantly antagonized the CCR9-mediated migration of the positively selected DP cells towards CCL25 in vitro. Based on these data, the authors proposed the following model: CCR9 expression on DP thymocytes retains them in the cortex in close proximity to sources of CCL25. Positive selection up-regulates expression of plexinD1, which diminishes the effect of CCL25 and allows the cells to respond to CCR7 ligands and migrate to the medulla. Intriguingly, Sema3E-deficient mice exhibit disturbed cortical and medullary organization due to the lack of clear delineation between DP and SP thymocyte localization at the corresponding regions. In addition, in contrast to localization of WT CD69+ thymocytes in the medulla or adjacent to the CMJ, Sema3E−/− CD69+ thymocytes were found outside of the medulla, suggesting that Sema3E plays a critical role in migration of positively selected or activated thymocytes. Similarly, CD69+ thymocytes from plexin D1−/− fetal liver-transplanted mice were also found outside of medulla and SP thymocytes formed ectopic medulla under the thymic capsule. This study demonstrated that Sema3E/plexinD1 interaction plays an important role in directing migration of maturing thymocytes by modulating chemotactic responses and provided a glimpse into the previously unappreciated roles of semaphorins and their receptors in the immune system.
Migration in the medulla
Once in the medulla, thymocytes have two major tasks to accomplish—thorough screening against auto-antigens to ensure that only self-tolerant cells will enter the circulation, and further maturation of thymocytes that bear TCRs potentially reactive with pathogens. How thymocytes prepare themselves to exit into the bloodstream is still incompletely understood, but the major events include tuning down the responsiveness of the TCR and regulating the expression of chemokine receptors and adhesion molecules to facilitate their egress and to guide them to the appropriate location outside the thymus [123]. The removal of the potentially auto-reactive thymocytes is known as negative selection and is thought to proceed mainly in the medulla. The thymic medulla provides a specialized environment for production, delivery, and presentation of numerous tissue-restricted self-antigens. There are at least five distinct pathways through which antigens are presented to the SP thymocytes [124]: (1) constitutive production of ubiquitously expressed and thymus-specific proteins and their presentation through the classical MHC I and MHC II pathways; (2) non-classical sampling of intracellular proteins through autophagy [125]; (3) delivery of blood-borne antigens to the medullary DCs [126–128]; (4) import of extrathymic antigens by circulating DCs [129]; (5) promiscuous gene expression (pGE) of tissue-restricted antigens (TRAs) by mTECs [130]. The last mechanism has received a lot of attention recently following the discovery of the transcriptional factor AutoImmune REgulator (AIRE) and the autoimmune disease occurring in patients with AIRE mutations or AIRE-deficient mice [131–133]. AIRE is expressed by a subset of mature mTECs and drives the stochastic expression of genes considered to be strictly restricted to peripheral tissues. The only known function of these proteins in mTECs is to be presented on MHC molecules for the purposes of negative selection. The molecular details of this pathway are still poorly understood, although some fascinating details of how AIRE might work are starting to emerge [134].
Recently, compelling evidence was obtained that conventional TCRαβ thymocytes spend only 4–5 d in the medulla [135]. This short stay further complicates the task for the SP thymocytes to search for the rare, promiscuously expressed peripheral tissue antigens regulated by AIRE [136]. Nevertheless, the process of negative selection is remarkably efficient. Apparently, the scanning mechanism employed by SP thymocytes is sufficiently rapid and complete that essentially each of them encounters the full collection of TRAs. That raises the questions: What is the strategy that thymocytes employ to become “acquainted” with the rare self-antigens and what are the guidance cues from the medullary environment that allow them to do so?
The guidance cues for thymocytes in the medulla are most likely the same that attract them to that site. However, no systematic studies have been conducted to test the role of different chemokines and their receptors in regulating thymocyte motility and development in the medulla. Based on expression data of chemokine receptors, we can speculate that semi-mature CD4SP thymocytes will be attracted to CCR4 and CCR9 ligands; CD8SP will be attracted to CCR9 ligands; almost all medullary thymocytes will be attracted to CCR7 ligands; and only the most mature SP thymocytes will be attracted to S1P (see below). However, to build a complete picture of the chemokine-dependent motility in the medulla, the exact distribution of the chemokines in the medulla needs to be determined. Our only glimpse into this problem comes from the study by Ritchie-Ehrilch et al. [113], which reported that CCR7−/− CD4SP migrate more slowly than WT, suggesting that CCR7 ligands contribute to the motility of medullary thymocytes. It will be informative to determine what roles CCR4 and CCR9 play in SP thymocyte motility in the medulla.
Determining thymocyte motility in the medulla using two-photon microscopy
The in vivo migration pattern of the medullary thymocytes has been described only recently [122, 137]. The major obstacle to observe the migratory behavior of cells in the medulla is that the medulla is typically located inside of the thymus covered by a thick layer of cortex, and is often beyond the range of two-photon microscopy. To circumvent this problem, the thymus needs to be sliced to expose the medulla for direct imaging of SP thymocytes [1, 113, 122]. Using this approach, we recently characterized the behavior of the cells in the medulla and found that TCR transgenic thymocytes that are not subject to negative selection move rapidly inside the medulla (Fig. 5). Their average speeds can exceed 15 μm/min, which makes them the fastest of any cell type in a 3D environment. Another striking difference compared to the cortical DP thymocytes is that the medullary thymocytes seem to be restricted to a certain region instead of following a random walk pattern of motility [93, 113].
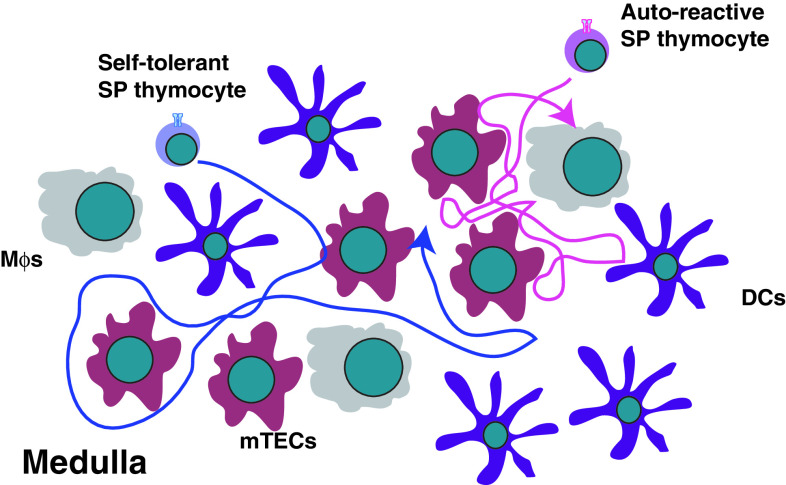
Fig. 5.
Distinct migration patterns of self-tolerant and auto-reactive SP thymocytes within the medulla. Self-tolerant SP thymocytes migrate at high speeds making numerous contacts with the resident stromal cells. In contrast, auto-reactive SP thymocytes move slowly in confinement zones making slightly prolonged contacts with DCs. mTEC medullary thymic epithelial cells, DC dendritic cells, Mϕ macrophages
Interestingly, two-photon microscopy studies in the OT I/RIPmOva double transgenic system revealed that auto-reactive CD8SP thymocytes behave in a markedly different way than self-tolerant cells. OT I TCR transgenic thymocytes recognize a peptide derived from ovalbumin and RIPmOva transgenic mice express ovalbumin in the thymic medulla [122]. As a result, auto-reactive OT I thymocytes are efficiently deleted in an AIRE-dependent manner in the double-transgenic mice [138]. Visualization of migratory behaviors of auto-reactive OT I cells using two-photon microscopy showed that OT I cells moved significantly slower in the presence of RIPmOva (Fig. 5). Moreover, the auto-reactive OT I thymocytes migrated in much smaller confinement zones (~20–30 μm) compared to non-autoreactive cells. The cells stayed in their zones for a long period of time and only rarely entered or left the zone. One attractive hypothesis is that the zone is defined by the presence of a RIPmOva expressing mTEC that has “spilled” ovalbumin onto neighboring cells. Then the whole group of cells acts as an antigen-presenting zone for ovalbumin-specific thymocytes.
Quite interestingly, polyclonal medullary thymocytes can be divided into two groups based on their speeds. The majority of the wild-type cells are fast and slightly confined, resembling OT I in the absence of their negative selecting ligand [122]. CD8SP cells seem to be a little bit faster than CD4SP. FoxP3-expressing Tregs are indistinguishable from the conventional CD4SP cells in motility parameters. However, a population of ~20–30% of all medullary thymocytes migrates similar to the auto-reactive OT I cells (i.e., in a slow and more confined manner). Since both CD4SP and CD8SP thymocytes are present among these slow cells, it is unlikely that CD4/CD8 lineage differences dictate the appearance of this population. It is more likely that these cells are undergoing negative selection and are slowing down because of interactions with their cognate self-antigens in the confinement zones.
The composition of the confinement zones for negative selection is unknown. However, their size (~20–30 μm) is consistent with the size of a large cell (e.g., mTEC or DC), so it is possible that the auto-reactive cells crawl on the surface of an antigen-presenting cell and interact with it constantly. However, the OT I thymocytes in a RIPmOva host do not predominantly interact with a single DC, but instead engage in serial contacts with multiple DCs [122]. How the auto-reactive cells interact with mTECs, especially the AIRE-expressing subset, is unknown. Overall, the interaction pattern of OT I cells in RIPmOva host suggests that the auto-reactive thymocytes can integrate multiple stimuli by engaging in serial interactions with antigen-presenting cells before they commit to death.
Thymic egress
The last migratory event that thymocytes complete is egress from the thymus. It has been known for a long time that ~1% of all thymocytes are exported every day into the circulation (~106 in mice [139], 107–108 depending on the age in humans [140]), however, the molecular details of thymic egress emerged only recently. Two pharmacological agents played a crucial role in the study of the thymic egress. The first clue about the nature of the signaling pathway that leads to thymic egress came from studies utilizing PTx [141, 142]. Thymic egress was blocked when PTx was expressed in thymocytes, implying that a chemokine-induced signaling pathway plays an important role in the process [142].
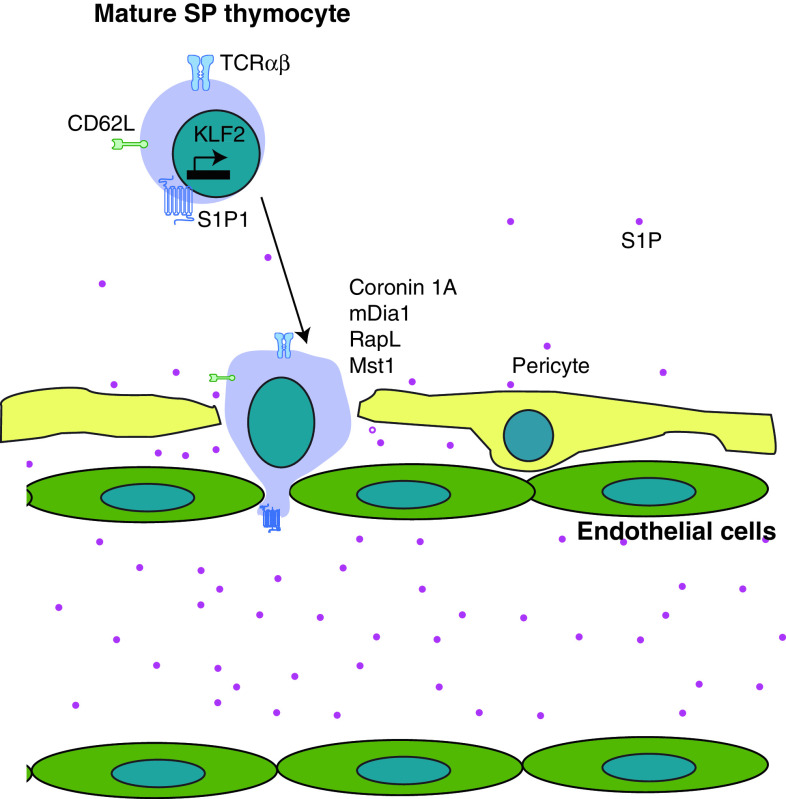
The identity of the chemokine receptor that mediates thymic egress remained a mystery for many years. The breakthrough came when the small molecule FTY720, identified in a screen for immunosuppressive drugs, was shown to inhibit the exit of thymocytes into circulation [143]. The effect of FTY720 is via the sphingosine-1-phosphate receptor 1 (S1P1), revealing that S1P1 and its ligand S1P are critical for thymic egress [144, 145]. The receptor is expressed on the most mature SP thymocytes. Genetic manipulations of the enzymes that convert S1P to its active form (S1P kinases) and the enzymes that degrade it (S1P lyases) confirmed the proposed central role of this chemokine/receptor pair for thymic egress [146, 147]. In addition, overexpression of S1P1 on DP thymocytes caused them to exit the thymus, verifying that S1P1 expression is sufficient for thymic emigration [148]. The following model was proposed based on these data (Fig. 6): thymocytes follow an S1P gradient that is established by the balance of its production and degradation. The S1P lyase is very active in the parenchyma of the organ, maintaining S1P at a low concentration. In the bloodstream, there is an abundance of S1P that is produced mainly by hematopoietic cells. However, this model still could not explain bone marrow chimera experiments, which showed that the smaller pool of S1P produced by radiation-resistant stromal cells is more important for the egress from the thymus than the larger pool of S1P produced by hematopoietic cells. This conundrum was solved recently by Zachariah and Cyster, who showed that the blood vessel-ensheathing pericytes are a critical source of S1P and thymic egress is completely dependent upon the production of S1P by these cells (Fig. 6) [148].
Fig. 6.
Egress from the thymus. Mature SP thymocytes express CD62L and the chemokine receptor S1P1 that are transcriptionally regulated by KLF2. They are attracted to S1P produced by pericytes and blood cells and transmigrate across the endothelial layer to enter the circulation. Cytoskeleton remodeling molecules such as Coronin 1A and mDia1 as well as small GTPase interacting molecules such as RapL and Mst1 are essential for the transmigration
One of the important regulators of S1P1 is CD69, better known as a marker for T cell activation. Although the details are still being investigated, it appears that CD69 and S1P1 mutually antagonize the expression of each other on the surface of T cells [149]. In the thymus, CD69 is expressed on the semi-mature SP thymocytes that do not express S1P1. The further maturation of these cells is associated with loss of CD69 and gain of S1P1 expression that renders them competent to exit the thymus. In agreement with this view, CD69-deficient SP thymocytes stay in the thymus for a slightly shorter time than WT [148], perhaps due to premature expression of S1P1 on these cells in the absence of CD69.
As previously mentioned, the average time thymocytes spend in the medulla before they emigrate is 4–5 days [135]. The duration of this stay most likely depends on the factors that control the expression of the components of the S1P1 signaling pathway. So far, the only confirmed direct regulator of S1P1 expression is the transcription factor Kruppel Lung Factor 2 (KLF2) [150]. KLF2 appears to be a critical coordinator of the thymocyte maturation and exit because it also regulates the expression of other adhesion molecules such as CD62L and β7-integrin that are important for the migration into the secondary lymphoid organs and other peripheral tissues. Similarly to S1P1 deficiency, the ablation of KLF2 resulted in almost complete loss of mature T cells in the periphery, while SP thymocytes accumulated in the thymus. KLF2 itself seems to be under the control of FOXO1 transcription factor because the KLF2 mRNA level was decreased in cells deficient for FOXO1 [151]. However, whether this regulation is direct is unknown. Most likely, phosphatidylinositol-3-kinase (PI3K) is involved further upstream in this pathway, as constitutive activation of PI3K affects the egress of thymocytes from the thymus and down-regulates KLF2 expression [152].
Additional mechanisms for thymic egress that might be operational under certain circumstances have been proposed. For example, emigration of thymocytes in the neonatal period might be dependent on CCR7 as demonstrated using FTOC, although this chemokine receptor is clearly dispensable for egress from the adult thymus [153]. A role for chemorepulsion as a mechanism mediating intravasation has also been proposed. Specifically, the CXCR4-CXCL12 axis has been singled out by in vitro experiments in which CXCR4-expressing thymocytes were found to move away from the thymus in a CXCL12-dependent manner [154]. In addition, antagonizing CXCL12 in neonatal mice led to an accumulation of CD4SP in the thymus. Another pathway controlled by the transcription factor FoxJ1 has been proposed to regulate thymic egress [155]. Overexpression of FoxJ1 inhibited emigration of mature thymocytes, although they still express functional S1P1. The molecular details of this pathway remain unknown.
The chemokine signaling pathways regulating thymic egress ultimately converge on the cytoskeletal components to mediate the reverse transmigration of the mature thymocytes through the blood vessel wall. Several cytoskeletal proteins are required for this process. Coronin 1A is mutated in rare patients with severe combined immunodeficiency and mutant mice for this protein have a defect in the thymic egress [156]. The function of Coronin 1A is to inhibit F-actin branching assembly controlled by the Arp2/3 complex. Thus, Coronin 1A-deficient cells accumulate more F-actin. Another actin regulator, mDia1, also plays a role in the emigration of thymocytes as evidenced by the accumulation of mature thymocytes in the thymus of mDia1−/− mice [157]. mDia1, in contrast to Arp2/3, promotes the formation of unbranched F-actin fibers. Thus, it is likely that the formation of both branched and unbranched actin filaments is important for the proper cytoskeleton remodeling during reverse transmigration of thymocytes. In addition, the actin-bundling protein L-plastin was also recently shown to be involved in the exit from the thymus [158]. Although the expression of S1P1 was not affected on CD4SP L-plastin-deficient thymocytes, their migration towards S1P was severely impaired. As a consequence, mature thymocytes failed to egress and accumulated in the L-plastin-deficient thymus.
A potential connection between the chemokine receptors and cytoskeleton remodeling involves the Ras/Rho family of small GTPases that are often regulated by chemokines. RapL, an effector of the small GTPase Rap1, is required for lymphocyte adhesion and cell polarization triggered by chemokines. Indeed, RapL-deficient mice have a defect in egress from the thymus [159]. Another molecule that associates with RapL, Mst1, is critically required for this process as well [160]. Although the details of the signaling pathways triggered by S1P are beginning to be identified, it is still unclear how the small GTPases and their associated proteins are regulated by S1P and how their actions are transmitted to the actin cytoskeleton.
The place where thymocytes exit has been a matter of debate, but emerging evidence favors the large blood vessels at the CMJ as the primary location of the egress. While some studies have suggested that lymphatic vessels might play a role in this process [161], very recent work by Jason Cyster’s group showed that this is unlikely to be the case. The authors demonstrated that thymocytes in the act of egress could be observed using a clever intravascular labeling technique. Moreover, they showed that the number of cells exiting from the postcapillary venules at the CMJ closely matches the thymic output calculated using other methods [148]. Furthermore, abolishing S1P production by the lymphatic endothelium had no effect on the rate of the cells exiting the thymus. While the physiological places for egress are the large vessels at the CMJ, thymocytes can exit from other locations as well. For example, DP cells overexpressing S1P1 accumulate around and intravasate into the cortical blood vessels.
Although the above data describe a fascinating model for thymic egress, it is important to remember that real-time imaging of thymic egress has not been achieved yet. This is mainly due to the fact that all two-photon imaging experiments of the thymus have been done on explants. If blood flow has to be preserved in order for thymocytes to intravasate, other approaches such as thymus transplantation under the kidney capsule must be employed.
Concluding remarks
Although much progress has been made studying migration in the thymus, there are still many challenging questions to be answered. For example, the identity of TSPs (thymic settling progenitor cells) is still not clear. According to previous studies, recruitment of these rare cells required both chemokine receptors and adhesion molecules. Mice doubly deficient for both CCR7 and CCR9, or deficient for PSGL-1, exhibited a dramatic reduction in the number of ETPs, suggesting that recruitment of TSPs was inhibited. However, it is also possible that these factors are involved in survival and proliferation of ETPs. In a similar vein, it is still unknown what specific signals the thymus uses to intermittently recruit TSPs from the blood, and among those signals, which factors function as thymic gatekeepers to control thymic cellularity.
Although some details of thymocyte migration have been elucidated, there is still much to be learned to fully explain the relationship between thymocyte migration and development. One of the remaining questions is what factors determine thymocyte migration within the cortex. For instance, DN thymocytes migrate outwards towards the SCZ in response to CCL25 and possibly CXCL12, while DP thymocytes migrate through the cortex in response to, most likely, the same chemokines. Therefore, there must be other factors involved in the regulation of the long-range thymocyte migration. A recent report demonstrated that gene expression profiles of stromal cells from spatially distinct thymic regions (including the medulla, the perimedullary cortex and the SCZ) are indeed different [162]. The only exception is the central cortex region that lacks a unique signature of its own. The authors of this paper suggest that the medulla, the perimedullary cortex, and the SCZ provide specialized inductive microenvironments to developing thymocytes, and the central cortex is likely to modulate the proximity of progenitors moving between the perimedullary cortex and the SCZ. The next challenges will be to identify the exact cell types that thymocytes interact with at different developmental stages within these distinct microenvironments and to determine what chemoattractants, chemorepellents, and adhesion substrates these cells express.
Once they reach a specific microenvironment rich in chemokines-decorated stromal cells, the developing thymocytes need to be released from the chemokine signals and relocate to another microenvironment. The signals that regulate this release remain unclear. Although studies using two-photon microscopy made it possible to visualize dynamic interaction and migration behaviors of thymocytes in the cortex and medulla, there are still many areas that have not benefited from these methods. For example, it is noteworthy that the outward migration of DN thymocytes in vivo has not been visualized due to the difficulties of marking only DN thymocytes in the intact thymus and their limited numbers. An experiment overlaying sorted DN thymocytes onto thymic slices has been performed, however the outward migration of the DN thymocytes has not been reported [137]. Furthermore, as previously mentioned, what attractants guide mature SP thymocytes to efficiently scan the medullary environment for self-antigens still remains to be determined.
CCR7 has been identified as an essential chemokine receptor that induces migration of positively selected thymocytes from the cortex to the medulla. However, recent findings revealed that, although CCR7-deficient thymocytes have not shown a preference to migrate to the medulla like WT thymocytes, they were able to cross the CMJ and localize to the medulla. These results indicate that other factors are involved in CMJ transmigration. Adhesion to a substrate has been proposed to mediate this process, but the identity of this substrate is unknown at present. In addition, the roles of CCR4 and CCR9 in migration from the cortex to the medulla have not been investigated.
Since thymic education occurs through interaction between thymocytes and stromal cells, the molecular details of this process require further investigation. As shown in the case of GIT2−/− thymocytes, defects in thymocyte motility result in inefficient positive selection. There are many molecular components involved in thymocyte motility and interaction, and it is possible that aberrations in these pathways influence thymic positive and negative selection. The molecular mechanisms that regulate SP thymocyte motility and negative selection have not been identified yet, but it will be interesting to define whether abnormalities of SP thymocyte motility and interaction with mTECs will lead to inappropriate negative selection and autoimmunity. If these factors indeed influence thymic selection, that would require the selection models to incorporate parameters of motility in addition to TCR affinity and identity of the selecting cells.
In conclusion, the intrinsic thymocyte motility and their responsiveness to guidance signals determine their journey within the thymus during their maturation. Factors influencing thymocyte motility affect thymic selection and other important developmental steps. Proper thymocyte development is fundamental not only for achieving immunity against pathogens or cancer, it is also crucial for protection from autoimmunity. Controlling thymocyte seeding, migration within the thymus, and egress may benefit the treatment of diseases. For example, patients suffering from acquired T cell deficiencies (AIDS) or after stem cell transplantation will benefit from a treatment to boost TSPs into the thymus to restore a mature T cell compartment [163–165]. Furthermore, manipulating the egress of thymocytes or lymphocytes holds promise in treating autoimmune diseases by preventing new self-reactive clones from entering the circulation [144, 166–168]. However, much caution needs to be paid in the strategy to block migration of autoimmune T cells by using antibodies targeting integrins or chemokine receptors as these treatments might disturb thymocyte migration within the thymus, ultimately affecting positive or negative selection.
Acknowledgments
We thank E. Robey, J. Ross, B. Au-Yeung, and A. Limlander for reading the manuscript and providing comments. Supported by Grant Number K01 AR059754-01 from NIAMS (H.P.) and the Cancer Research Institute (I.D.).
References
- 1.Bhakta NR, Oh DY, Lewis RS. Calcium oscillations regulate thymocyte motility during positive selection in the three-dimensional thymic environment. Nat Immunol. 2005;6(2):143–151. doi: 10.1038/ni1161. [DOI] [PubMed] [Google Scholar]
- 2.Petrie HT, Zúñiga-Pflücker JC. Zoned out: functional mapping of stromal signaling microenvironments in the thymus. Annu Rev Immunol. 2007;25:649–679. doi: 10.1146/annurev.immunol.23.021704.115715. [DOI] [PubMed] [Google Scholar]
- 3.Boehm T, Bleul CC. Thymus-homing precursors and the thymic microenvironment. Trends Immunol. 2006;27(10):477–484. doi: 10.1016/j.it.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 4.Raviola E, Karnovsky MJ. Evidence for a blood–thymus barrier using electron-opaque tracers. J Exp Med. 1972;136(3):466–498. doi: 10.1084/jem.136.3.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bousso P, Robey EA. Dynamic behavior of T cells and thymocytes in lymphoid organs as revealed by two-photon microscopy. Immunity. 2004;21(3):349–355. doi: 10.1016/j.immuni.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 6.Zlotnik A, Yoshie O. Chemokines: a new classification system and their role in immunity. Immunity. 2000;12(2):121–127. doi: 10.1016/s1074-7613(00)80165-x. [DOI] [PubMed] [Google Scholar]
- 7.Janas ML, Turner M. Stromal cell-derived factor 1α and CXCR4: newly defined requirements for efficient thymic β-selection. Trends Immunol. 2010;31(10):370–376. doi: 10.1016/j.it.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 8.Petrie RJ, Doyle AD, Yamada KM. Random versus directionally persistent cell migration. Nat Rev Mol Cell Biol. 2009;10(8):538–549. doi: 10.1038/nrm2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilkinson PC. Chemotaxis and chemokinesis: confusion about definitions. J Immunol Methods. 1988;110(1):143–149. doi: 10.1016/0022-1759(88)90094-4. [DOI] [PubMed] [Google Scholar]
- 10.Wilkinson P. Cell locomotion and chemotaxis: basic concepts and methodological approaches. Methods. 1996;10(1):74–81. doi: 10.1006/meth.1996.0081. [DOI] [PubMed] [Google Scholar]
- 11.Pasquale EB. Eph-ephrin bidirectional signaling in physiology and disease. Cell. 2008;133(1):38–52. doi: 10.1016/j.cell.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 12.Vergara-Silva A, Schaefer KL, Berg LJ. Compartmentalized Eph receptor and ephrin expression in the thymus. Mech Dev. 2002;119(Suppl 1):S225–S229. doi: 10.1016/s0925-4773(03)00121-7. [DOI] [PubMed] [Google Scholar]
- 13.Munoz JJ, Alonso CL, Sacedon R, Crompton T, Vicente A, Jimenez E, Varas A, Zapata AG. Expression and function of the Eph A receptors and their ligands ephrins A in the rat thymus. J Immunol. 2002;169(1):177–184. doi: 10.4049/jimmunol.169.1.177. [DOI] [PubMed] [Google Scholar]
- 14.Alfaro D, Garcia-Ceca JJ, Cejalvo T, Jimenez E, Jenkinson EJ, Anderson G, Munoz JJ, Zapata A. EphrinB1-EphB signaling regulates thymocyte-epithelium interactions involved in functional T cell development. Eur J Immunol. 2007;37(9):2596–2605. doi: 10.1002/eji.200737097. [DOI] [PubMed] [Google Scholar]
- 15.Munoz JJ, Alfaro D, Garcia-Ceca J, Alonso CL, Jimenez E, Zapata A. Thymic alterations in EphA4-deficient mice. J Immunol. 2006;177(2):804–813. doi: 10.4049/jimmunol.177.2.804. [DOI] [PubMed] [Google Scholar]
- 16.Yu G, Mao J, Wu Y, Luo H, Wu J. Ephrin-B1 is critical in T-cell development. J Biol Chem. 2006;281(15):10222–10229. doi: 10.1074/jbc.M510320200. [DOI] [PubMed] [Google Scholar]
- 17.Foster KE, Gordon J, Cardenas K, Veiga-Fernandes H, Makinen T, Grigorieva E, Wilkinson DG, Blackburn CC, Richie E, Manley NR, Adams RH, Kioussis D, Coles MC. EphB-ephrin-B2 interactions are required for thymus migration during organogenesis. Proc Natl Acad Sci USA. 2010;107(30):13414–13419. doi: 10.1073/pnas.1003747107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shimoyama M, Matsuoka H, Nagata A, Iwata N, Tamekane A, Okamura A, Gomyo H, Ito M, Jishage K, Kamada N, Suzuki H, Tetsuo Noda T, Matsui T. Developmental expression of EphB6 in the thymus: lessons from EphB6 knockout mice. Biochem Biophys Res Commun. 2002;298(1):87–94. doi: 10.1016/s0006-291x(02)02399-9. [DOI] [PubMed] [Google Scholar]
- 19.Alfaro D, Munoz JJ, Garcia-Ceca J, Cejalvo T, Jimenez E, Zapata AG (2011) The Eph/ephrinB signal balance determines the pattern of T-cell maturation in the thymus. Immunol Cell Biol. doi:10.1038/icb.2010.172 [DOI] [PubMed]
- 20.Garcia-Ceca J, Jimenez E, Alfaro D, Cejalvo T, Munoz JJ, Zapata AG. Cell-autonomous role of EphB2 and EphB3 receptors in the thymic epithelial cell organization. Eur J Immunol. 2009;39(10):2916–2924. doi: 10.1002/eji.200939437. [DOI] [PubMed] [Google Scholar]
- 21.de Wit J, Verhaagen J. Role of semaphorins in the adult nervous system. Prog Neurobiol. 2003;71(2–3):249–267. doi: 10.1016/j.pneurobio.2003.06.001. [DOI] [PubMed] [Google Scholar]
- 22.Okuno T, Nakatsuji Y, Kumanogoh A (2011) The role of immune semaphorins in multiple sclerosis. FEBS Lett. doi:10.1016/j.febslet.2011.03.033 [DOI] [PubMed]
- 23.Takamatsu H, Okuno T, Kumanogoh A. Regulation of immune cell responses by semaphorins and their receptors. Cell Mol Immunol. 2010;7(2):83–88. doi: 10.1038/cmi.2009.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kolodkin AL, Matthes DJ, Goodman CS. The semaphorin genes encode a family of transmembrane and secreted growth cone guidance molecules. Cell. 1993;75(7):1389–1399. doi: 10.1016/0092-8674(93)90625-z. [DOI] [PubMed] [Google Scholar]
- 25.Kruger RP, Aurandt J, Guan KL. Semaphorins command cells to move. Nat Rev Mol Cell Biol. 2005;6(10):789–800. doi: 10.1038/nrm1740. [DOI] [PubMed] [Google Scholar]
- 26.Mendes-da-Cruz DA, Lepelletier Y, Brignier AC, Smaniotto S, Renand A, Milpied P, Dardenne M, Hermine O, Savino W. Neuropilins, semaphorins, and their role in thymocyte development. Ann N Y Acad Sci. 2009;1153:20–28. doi: 10.1111/j.1749-6632.2008.03980.x. [DOI] [PubMed] [Google Scholar]
- 27.Lepelletier Y, Smaniotto S, Hadj-Slimane R, Villa-Verde DM, Nogueira AC, Dardenne M, Hermine O, Savino W. Control of human thymocyte migration by Neuropilin-1/Semaphorin-3A-mediated interactions. Proc Natl Acad Sci USA. 2007;104(13):5545–5550. doi: 10.1073/pnas.0700705104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hogg N, Patzak I, Willenbrock F. The insider’s guide to leukocyte integrin signalling and function. Nat Rev Immunol. 2011;11(6):416–426. doi: 10.1038/nri2986. [DOI] [PubMed] [Google Scholar]
- 29.Woolf E, Grigorova I, Sagiv A, Grabovsky V, Feigelson SW, Shulman Z, Hartmann T, Sixt M, Cyster JG, Alon R. Lymph node chemokines promote sustained T lymphocyte motility without triggering stable integrin adhesiveness in the absence of shear forces. Nat Immunol. 2007;8(10):1076–1085. doi: 10.1038/ni1499. [DOI] [PubMed] [Google Scholar]
- 30.Lammermann T, Bader BL, Monkley SJ, Worbs T, Wedlich-Soldner R, Hirsch K, Keller M, Forster R, Critchley DR, Fassler R, Sixt M. Rapid leukocyte migration by integrin-independent flowing and squeezing. Nature. 2008;453(7191):51–55. doi: 10.1038/nature06887. [DOI] [PubMed] [Google Scholar]
- 31.Schmeissner PJ, Xie H, Smilenov LB, Shu F, Marcantonio EE. Integrin functions play a key role in the differentiation of thymocytes in vivo. J Immunol. 2001;167(7):3715–3724. doi: 10.4049/jimmunol.167.7.3715. [DOI] [PubMed] [Google Scholar]
- 32.Arroyo AG, Yang JT, Rayburn H, Hynes RO. Differential requirements for alpha4 integrins during fetal and adult hematopoiesis. Cell. 1996;85(7):997–1008. doi: 10.1016/s0092-8674(00)81301-x. [DOI] [PubMed] [Google Scholar]
- 33.Arroyo AG, Yang JT, Rayburn H, Hynes RO. Alpha4 integrins regulate the proliferation/differentiation balance of multilineage hematopoietic progenitors in vivo. Immunity. 1999;11(5):555–566. doi: 10.1016/s1074-7613(00)80131-4. [DOI] [PubMed] [Google Scholar]
- 34.Schmits R, Kundig TM, Baker DM, Shumaker G, Simard JJ, Duncan G, Wakeham A, Shahinian A, van der Heiden A, Bachmann MF, Ohashi PS, Mak TW, Hickstein DD. LFA-1-deficient mice show normal CTL responses to virus but fail to reject immunogenic tumor. J Exp Med. 1996;183(4):1415–1426. doi: 10.1084/jem.183.4.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Semmrich M, Smith A, Feterowski C, Beer S, Engelhardt B, Busch DH, Bartsch B, Laschinger M, Hogg N, Pfeffer K, Holzmann B. Importance of integrin LFA-1 deactivation for the generation of immune responses. J Exp Med. 2005;201(12):1987–1998. doi: 10.1084/jem.20041850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prockop SE, Petrie HT. Functional assessment of alphaEbeta7/E-cadherin interactions in the steady state postnatal thymus. Clin Dev Immunol. 2004;11(2):135–141. doi: 10.1080/10446670410001722212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bungartz G, Stiller S, Bauer M, Muller W, Schippers A, Wagner N, Fassler R, Brakebusch C. Adult murine hematopoiesis can proceed without beta1 and beta7 integrins. Blood. 2006;108(6):1857–1864. doi: 10.1182/blood-2005-10-007658. [DOI] [PubMed] [Google Scholar]
- 38.Scharffetter-Kochanek K, Lu H, Norman K, van Nood N, Munoz F, Grabbe S, McArthur M, Lorenzo I, Kaplan S, Ley K, Smith CW, Montgomery CA, Rich S, Beaudet AL. Spontaneous skin ulceration and defective T cell function in CD18 null mice. J Exp Med. 1998;188(1):119–131. doi: 10.1084/jem.188.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shier P, Otulakowski G, Ngo K, Panakos J, Chourmouzis E, Christjansen L, Lau CY, Fung-Leung WP. Impaired immune responses toward alloantigens and tumor cells but normal thymic selection in mice deficient in the beta2 integrin leukocyte function-associated antigen-1. J Immunol. 1996;157(12):5375–5386. [PubMed] [Google Scholar]
- 40.van der Neut R, Krimpenfort P, Calafat J, Niessen CM, Sonnenberg A. Epithelial detachment due to absence of hemidesmosomes in integrin beta 4 null mice. Nat Genet. 1996;13(3):366–369. doi: 10.1038/ng0796-366. [DOI] [PubMed] [Google Scholar]
- 41.Xu H, Gonzalo JA, St Pierre Y, Williams IR, Kupper TS, Cotran RS, Springer TA, Gutierrez-Ramos JC. Leukocytosis and resistance to septic shock in intercellular adhesion molecule 1-deficient mice. J Exp Med. 1994;180(1):95–109. doi: 10.1084/jem.180.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sligh JE, Jr, Ballantyne CM, Rich SS, Hawkins HK, Smith CW, Bradley A, Beaudet AL. Inflammatory and immune responses are impaired in mice deficient in intercellular adhesion molecule 1. Proc Natl Acad Sci USA. 1993;90(18):8529–8533. doi: 10.1073/pnas.90.18.8529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Koni PA, Joshi SK, Temann UA, Olson D, Burkly L, Flavell RA. Conditional vascular cell adhesion molecule 1 deletion in mice: impaired lymphocyte migration to bone marrow. J Exp Med. 2001;193(6):741–754. doi: 10.1084/jem.193.6.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zlotoff DA, Sambandam A, Logan TD, Bell JJ, Schwarz BA, Bhandoola A. CCR7 and CCR9 together recruit hematopoietic progenitors to the adult thymus. Blood. 2010;115(10):1897–1905. doi: 10.1182/blood-2009-08-237784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Donskoy E, Goldschneider I. Thymocytopoiesis is maintained by blood-borne precursors throughout postnatal life. A study in parabiotic mice. J Immunol. 1992;148(6):1604–1612. [PubMed] [Google Scholar]
- 46.Goldschneider I, Komschlies KL, Greiner DL. Studies of thymocytopoiesis in rats and mice I Kinetics of appearance of thymocytes using a direct intrathymic adoptive transfer assay for thymocyte precursors. J Exp Med. 1986;163(1):1–17. doi: 10.1084/jem.163.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Scollay R, Smith J, Stauffer V. Dynamics of early T cells: prothymocyte migration and proliferation in the adult mouse thymus. Immunol Rev. 1986;91:129–157. doi: 10.1111/j.1600-065x.1986.tb01487.x. [DOI] [PubMed] [Google Scholar]
- 48.Shortman K, Wu L. Early T lymphocyte progenitors. Annu Rev Immunol. 1996;14:29–47. doi: 10.1146/annurev.immunol.14.1.29. [DOI] [PubMed] [Google Scholar]
- 49.Foss DL, Donskoy E, Goldschneider I. The importation of hematogenous precursors by the thymus is a gated phenomenon in normal adult mice. J Exp Med. 2001;193(3):365–374. doi: 10.1084/jem.193.3.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bhandoola A, von Boehmer H, Petrie HT, Zúñiga-Pflücker JC. Commitment and developmental potential of extrathymic and intrathymic T cell precursors: plenty to choose from. Immunity. 2007;26(6):678–689. doi: 10.1016/j.immuni.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 51.Bell JJ, Bhandoola A. The earliest thymic progenitors for T cells possess myeloid lineage potential. Nature. 2008;452(7188):764–767. doi: 10.1038/nature06840. [DOI] [PubMed] [Google Scholar]
- 52.Allman D, Sambandam A, Kim S, Miller JP, Pagan A, Well D, Meraz A, Bhandoola A. Thymopoiesis independent of common lymphoid progenitors. Nat Immunol. 2003;4(2):168–174. doi: 10.1038/ni878. [DOI] [PubMed] [Google Scholar]
- 53.Moore MA, Owen JJ. Experimental studies on the development of the thymus. J Exp Med. 1967;126(4):715–726. doi: 10.1084/jem.126.4.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Haynes BF, Heinly CS. Early human T cell development: analysis of the human thymus at the time of initial entry of hematopoietic stem cells into the fetal thymic microenvironment. J Exp Med. 1995;181(4):1445–1458. doi: 10.1084/jem.181.4.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu C, Saito F, Liu Z, Lei Y, Uehara S, Love P, Lipp M, Kondo S, Manley N, Takahama Y. Coordination between CCR7- and CCR9-mediated chemokine signals in prevascular fetal thymus colonization. Blood. 2006;108(8):2531–2539. doi: 10.1182/blood-2006-05-024190. [DOI] [PubMed] [Google Scholar]
- 56.Krueger A, Willenzon S, Lyszkiewicz M, Kremmer E, Förster R. CC chemokine receptor 7 and 9 double-deficient hematopoietic progenitors are severely impaired in seeding the adult thymus. Blood. 2010;115(10):1906–1912. doi: 10.1182/blood-2009-07-235721. [DOI] [PubMed] [Google Scholar]
- 57.Stimamiglio MA, Jimenez E, Silva-Barbosa SD, Alfaro D, Garcia-Ceca JJ, Munoz JJ, Cejalvo T, Savino W, Zapata A. EphB2-mediated interactions are essential for proper migration of T cell progenitors during fetal thymus colonization. J Leukoc Biol. 2010;88(3):483–494. doi: 10.1189/jlb.0210079. [DOI] [PubMed] [Google Scholar]
- 58.Rossi FMV, Corbel SY, Merzaban JS, Carlow DA, Gossens K, Duenas J, So L, Yi L, Ziltener HJ. Recruitment of adult thymic progenitors is regulated by P-selectin and its ligand PSGL-1. Nat Immunol. 2005;6(6):626–634. doi: 10.1038/ni1203. [DOI] [PubMed] [Google Scholar]
- 59.Gossens K, Naus S, Corbel SY, Lin S, Rossi FMV, Kast J, Ziltener HJ. Thymic progenitor homing and lymphocyte homeostasis are linked via S1P-controlled expression of thymic P-selectin/CCL25. J Exp Med. 2009;206(4):761–778. doi: 10.1084/jem.20082502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Scimone ML, Aifantis I, Apostolou I, von Boehmer H, von Andrian UH. A multistep adhesion cascade for lymphoid progenitor cell homing to the thymus. Proc Natl Acad Sci USA. 2006;103(18):7006–7011. doi: 10.1073/pnas.0602024103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wu L, Kincade PW, Shortman K. The CD44 expressed on the earliest intrathymic precursor population functions as a thymus homing molecule but does not bind to hyaluronate. Immunol Lett. 1993;38(1):69–75. doi: 10.1016/0165-2478(93)90121-h. [DOI] [PubMed] [Google Scholar]
- 62.Protin U, Schweighoffer T, Jochum W, Hilberg F. CD44-deficient mice develop normally with changes in subpopulations and recirculation of lymphocyte subsets. J Immunol. 1999;163(9):4917–4923. [PubMed] [Google Scholar]
- 63.Shortman K, Egerton M, Spangrude GJ, Scollay R. The generation and fate of thymocytes. Semin Immunol. 1990;2(1):3–12. [PubMed] [Google Scholar]
- 64.Crompton T, Outram SV, Hager-Theodorides AL. Sonic hedgehog signalling in T-cell development and activation. Nat Rev Immunol. 2007;7(9):726–735. doi: 10.1038/nri2151. [DOI] [PubMed] [Google Scholar]
- 65.Schmitt TM, Zúñiga-Pflücker JC. Induction of T cell development from hematopoietic progenitor cells by delta-like-1 in vitro. Immunity. 2002;17(6):749–756. doi: 10.1016/s1074-7613(02)00474-0. [DOI] [PubMed] [Google Scholar]
- 66.Schmitt TM, Ciofani M, Petrie HT, Zúñiga-Pflücker JC. Maintenance of T cell specification and differentiation requires recurrent notch receptor-ligand interactions. J Exp Med. 2004;200(4):469–479. doi: 10.1084/jem.20040394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tan JB, Visan I, Yuan JS, Guidos CJ. Requirement for Notch1 signals at sequential early stages of intrathymic T cell development. Nat Immunol. 2005;6(7):671–679. doi: 10.1038/ni1217. [DOI] [PubMed] [Google Scholar]
- 68.Sambandam A, Maillard I, Zediak VP, Xu L, Gerstein RM, Aster JC, Pear WS, Bhandoola A. Notch signaling controls the generation and differentiation of early T lineage progenitors. Nat Immunol. 2005;6(7):663–670. doi: 10.1038/ni1216. [DOI] [PubMed] [Google Scholar]
- 69.Ciofani M, Zúñiga-Pflücker JC. Notch promotes survival of pre-T cells at the beta-selection checkpoint by regulating cellular metabolism. Nat Immunol. 2005;6(9):881–888. doi: 10.1038/ni1234. [DOI] [PubMed] [Google Scholar]
- 70.Plotkin J, Prockop SE, Lepique A, Petrie HT. Critical role for CXCR4 signaling in progenitor localization and T cell differentiation in the postnatal thymus. J Immunol. 2003;171(9):4521–4527. doi: 10.4049/jimmunol.171.9.4521. [DOI] [PubMed] [Google Scholar]
- 71.Trampont PC, Tosello-Trampont A-C, Shen Y, Duley AK, Sutherland AE, Bender TP, Littman DR, Ravichandran KS. CXCR4 acts as a costimulator during thymic beta-selection. Nat Immunol. 2010;11(2):162–170. doi: 10.1038/ni.1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zou YR, Kottmann AH, Kuroda M, Taniuchi I, Littman DR. Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature. 1998;393(6685):595–599. doi: 10.1038/31269. [DOI] [PubMed] [Google Scholar]
- 73.Ma Q, Jones D, Borghesani PR, Segal RA, Nagasawa T, Kishimoto T, Bronson RT, Springer TA. Impaired B-lymphopoiesis, myelopoiesis, and derailed cerebellar neuron migration in CXCR4- and SDF-1-deficient mice. Proc Natl Acad Sci USA. 1998;95(16):9448–9453. doi: 10.1073/pnas.95.16.9448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ara T, Itoi M, Kawabata K, Egawa T, Tokoyoda K, Sugiyama T, Fujii N, Amagai T, Nagasawa T. A role of CXC chemokine ligand 12/stromal cell-derived factor-1/pre-B cell growth stimulating factor and its receptor CXCR4 in fetal and adult T cell development in vivo. J Immunol. 2003;170(9):4649–4655. doi: 10.4049/jimmunol.170.9.4649. [DOI] [PubMed] [Google Scholar]
- 75.Kawabata K, Ujikawa M, Egawa T, Kawamoto H, Tachibana K, Iizasa H, Katsura Y, Kishimoto T, Nagasawa T. A cell-autonomous requirement for CXCR4 in long-term lymphoid and myeloid reconstitution. Proc Natl Acad Sci USA. 1999;96(10):5663–5667. doi: 10.1073/pnas.96.10.5663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Egawa T, Kawabata K, Kawamoto H, Amada K, Okamoto R, Fujii N, Kishimoto T, Katsura Y, Nagasawa T. The earliest stages of B cell development require a chemokine stromal cell-derived factor/pre-B cell growth-stimulating factor. Immunity. 2001;15(2):323–334. doi: 10.1016/s1074-7613(01)00185-6. [DOI] [PubMed] [Google Scholar]
- 77.Wurbel MA, Philippe JM, Nguyen C, Victorero G, Freeman T, Wooding P, Miazek A, Mattei MG, Malissen M, Jordan BR, Malissen B, Carrier A, Naquet P. The chemokine TECK is expressed by thymic and intestinal epithelial cells and attracts double- and single-positive thymocytes expressing the TECK receptor CCR9. Eur J Immunol. 2000;30(1):262–271. doi: 10.1002/1521-4141(200001)30:1<262::AID-IMMU262>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 78.Misslitz A, Pabst O, Hintzen G, Ohl L, Kremmer E, Petrie HT, Forster R. Thymic T cell development and progenitor localization depend on CCR7. J Exp Med. 2004;200(4):481–491. doi: 10.1084/jem.20040383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vicari AP, Figueroa DJ, Hedrick JA, Foster JS, Singh KP, Menon S, Copeland NG, Gilbert DJ, Jenkins NA, Bacon KB, Zlotnik A. TECK: a novel CC chemokine specifically expressed by thymic dendritic cells and potentially involved in T cell development. Immunity. 1997;7(2):291–301. doi: 10.1016/s1074-7613(00)80531-2. [DOI] [PubMed] [Google Scholar]
- 80.Norment AM, Bogatzki LY, Gantner BN, Bevan MJ. Murine CCR9, a chemokine receptor for thymus-expressed chemokine that is up-regulated following pre-TCR signaling. J Immunol. 2000;164(2):639–648. doi: 10.4049/jimmunol.164.2.639. [DOI] [PubMed] [Google Scholar]
- 81.Wurbel M-A, Malissen B, Campbell JJ. Complex regulation of CCR9 at multiple discrete stages of T cell development. Eur J Immunol. 2006;36(1):73–81. doi: 10.1002/eji.200535203. [DOI] [PubMed] [Google Scholar]
- 82.Benz C, Heinzel K, Bleul CC. Homing of immature thymocytes to the subcapsular microenvironment within the thymus is not an absolute requirement for T cell development. Eur J Immunol. 2004;34(12):3652–3663. doi: 10.1002/eji.200425248. [DOI] [PubMed] [Google Scholar]
- 83.Uehara S, Grinberg A, Farber JM, Love PE. A role for CCR9 in T lymphocyte development and migration. J Immunol. 2002;168(6):2811–2819. doi: 10.4049/jimmunol.168.6.2811. [DOI] [PubMed] [Google Scholar]
- 84.Holländer GA, Wang B, Nichogiannopoulou A, Platenburg PP, van Ewijk W, Burakoff SJ, Gutierrez-Ramos JC, Terhorst C. Developmental control point in induction of thymic cortex regulated by a subpopulation of prothymocytes. Nature. 1995;373(6512):350–353. doi: 10.1038/373350a0. [DOI] [PubMed] [Google Scholar]
- 85.van Ewijk W, Holländer G, Terhorst C, Wang B. Stepwise development of thymic microenvironments in vivo is regulated by thymocyte subsets. Development. 2000;127(8):1583–1591. doi: 10.1242/dev.127.8.1583. [DOI] [PubMed] [Google Scholar]
- 86.Klug DB, Carter C, Crouch E, Roop D, Conti CJ, Richie ER. Interdependence of cortical thymic epithelial cell differentiation and T-lineage commitment. Proc Natl Acad Sci USA. 1998;95(20):11822–11827. doi: 10.1073/pnas.95.20.11822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Robey EA, Bousso P. Visualizing thymocyte motility using 2-photon microscopy. Immunol Rev. 2003;195:51–57. doi: 10.1034/j.1600-065x.2003.00069.x. [DOI] [PubMed] [Google Scholar]
- 88.Ladi E, Yin X, Chtanova T, Robey EA. Thymic microenvironments for T cell differentiation and selection. Nat Immunol. 2006;7(4):338–343. doi: 10.1038/ni1323. [DOI] [PubMed] [Google Scholar]
- 89.Witt CM, Robey EA. Thymopoiesis in 4 dimensions. Semin Immunol. 2005;17(1):95–102. doi: 10.1016/j.smim.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 90.Yin X, Chtanova T, Ladi E, Robey EA. Thymocyte motility: mutants, movies and migration patterns. Curr Opin Immunol. 2006;18(2):191–197. doi: 10.1016/j.coi.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 91.Bhakta NR, Lewis RS. Real-time measurement of signaling and motility during T cell development in the thymus. Semin Immunol. 2005;17(6):411–420. doi: 10.1016/j.smim.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 92.Bousso P, Bhakta NR, Lewis RS, Robey E. Dynamics of thymocyte-stromal cell interactions visualized by two-photon microscopy. Science. 2002;296(5574):1876–1880. doi: 10.1126/science.1070945. [DOI] [PubMed] [Google Scholar]
- 93.Witt CM, Raychaudhuri S, Schaefer B, Chakraborty AK, Robey EA. Directed migration of positively selected thymocytes visualized in real time. PLoS Biol. 2005;3(6):e160. doi: 10.1371/journal.pbio.0030160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kurobe H, Liu C, Ueno T, Saito F, Ohigashi I, Seach N, Arakaki R, Hayashi Y, Kitagawa T, Lipp M, Boyd RL, Takahama Y. CCR7-dependent cortex-to-medulla migration of positively selected thymocytes is essential for establishing central tolerance. Immunity. 2006;24(2):165–177. doi: 10.1016/j.immuni.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 95.Ladi E, Schwickert TA, Chtanova T, Chen Y, Herzmark P, Yin X, Aaron H, Chan SW, Lipp M, Roysam B, Robey EA. Thymocyte-dendritic cell interactions near sources of CCR7 ligands in the thymic cortex. J Immunol. 2008;181(10):7014–7023. doi: 10.4049/jimmunol.181.10.7014. [DOI] [PubMed] [Google Scholar]
- 96.Phee H, Dzhagalov I, Mollenauer M, Wang Y, Irvine DJ, Robey E, Weiss A. Regulation of thymocyte positive selection and motility by GIT2. Nat Immunol. 2010;11(6):503–511. doi: 10.1038/ni.1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Turner CE. Paxillin interactions. J Cell Sci. 2000;113(Pt 23):4139–4140. doi: 10.1242/jcs.113.23.4139. [DOI] [PubMed] [Google Scholar]
- 98.Frank SR, Hansen SH. The PIX-GIT complex: a G protein signaling cassette in control of cell shape. Semin Cell Dev Biol. 2008;19(3):234–244. doi: 10.1016/j.semcdb.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Randazzo PA, Inoue H, Bharti S. Arf GAPs as regulators of the actin cytoskeleton. Biol Cell. 2007;99(10):583–600. doi: 10.1042/bc20070034. [DOI] [PubMed] [Google Scholar]
- 100.Hoefen RJ, Berk BC. The multifunctional GIT family of proteins. J Cell Sci. 2006;119(Pt 8):1469–1475. doi: 10.1242/jcs.02925. [DOI] [PubMed] [Google Scholar]
- 101.Sabe H, Onodera Y, Mazaki Y, Hashimoto S. ArfGAP family proteins in cell adhesion, migration and tumor invasion. Curr Opin Cell Biol. 2006;18(5):558–564. doi: 10.1016/j.ceb.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 102.Vitale N, Patton WA, Moss J, Vaughan M, Lefkowitz RJ, Premont RT. GIT proteins. A novel family of phosphatidylinositol 3, 4, 5-trisphosphate-stimulated GTPase-activating proteins for ARF6. J Biol Chem. 2000;275(18):13901–13906. doi: 10.1074/jbc.275.18.13901. [DOI] [PubMed] [Google Scholar]
- 103.Premont RT, Claing A, Vitale N, Perry SJ, Lefkowitz RJ. The GIT family of ADP-ribosylation factor GTPase-activating proteins functional diversity of GIT2 through alternative splicing. J Biol Chem. 2000;275(29):22373–22380. doi: 10.1074/jbc.275.29.22373. [DOI] [PubMed] [Google Scholar]
- 104.Akiyama T, Maeda S, Yamane S, Ogino K, Kasai M, Kajiura F, Matsumoto M, Inoue J. Dependence of self-tolerance on TRAF6-directed development of thymic stroma. Science. 2005;308(5719):248–251. doi: 10.1126/science.1105677. [DOI] [PubMed] [Google Scholar]
- 105.Burkly L, Hession C, Ogata L, Reilly C, Marconi LA, Olson D, Tizard R, Cate R, Lo D. Expression of relB is required for the development of thymic medulla and dendritic cells. Nature. 1995;373(6514):531–536. doi: 10.1038/373531a0. [DOI] [PubMed] [Google Scholar]
- 106.Laufer TM, DeKoning J, Markowitz JS, Lo D, Glimcher LH. Unopposed positive selection and autoreactivity in mice expressing class II MHC only on thymic cortex. Nature. 1996;383(6595):81–85. doi: 10.1038/383081a0. [DOI] [PubMed] [Google Scholar]
- 107.Davalos-Misslitz AC, Rieckenberg J, Willenzon S, Worbs T, Kremmer E, Bernhardt G, Forster R. Generalized multi-organ autoimmunity in CCR7-deficient mice. Eur J Immunol. 2007;37(3):613–622. doi: 10.1002/eji.200636656. [DOI] [PubMed] [Google Scholar]
- 108.Yin X, Ladi E, Chan SW, Li O, Killeen N, Kappes DJ, Robey EA. CCR7 expression in developing thymocytes is linked to the CD4 versus CD8 lineage decision. J Immunol. 2007;179(11):7358–7364. doi: 10.4049/jimmunol.179.11.7358. [DOI] [PubMed] [Google Scholar]
- 109.Singer A, Adoro S, Park JH. Lineage fate and intense debate: myths, models and mechanisms of CD4- versus CD8-lineage choice. Nat Rev. 2008;8(10):788–801. doi: 10.1038/nri2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Suzuki G, Sawa H, Kobayashi Y, Nakata Y, Nakagawa K, Uzawa A, Sakiyama H, Kakinuma S, Iwabuchi K, Nagashima K. Pertussis toxin-sensitive signal controls the trafficking of thymocytes across the corticomedullary junction in the thymus. J Immunol. 1999;162(10):5981–5985. [PubMed] [Google Scholar]
- 111.Kwan J, Killeen N. CCR7 directs the migration of thymocytes into the thymic medulla. J Immunol. 2004;172(7):3999–4007. doi: 10.4049/jimmunol.172.7.3999. [DOI] [PubMed] [Google Scholar]
- 112.Ueno T, Saito F, Gray DH, Kuse S, Hieshima K, Nakano H, Kakiuchi T, Lipp M, Boyd RL, Takahama Y. CCR7 signals are essential for cortex-medulla migration of developing thymocytes. J Exp Med. 2004;200(4):493–505. doi: 10.1084/jem.20040643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ehrlich LI, Oh DY, Weissman IL, Lewis RS. Differential contribution of chemotaxis and substrate restriction to segregation of immature and mature thymocytes. Immunity. 2009;31(6):986–998. doi: 10.1016/j.immuni.2009.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Nitta T, Nitta S, Lei Y, Lipp M, Takahama Y. CCR7-mediated migration of developing thymocytes to the medulla is essential for negative selection to tissue-restricted antigens. Proc Natl Acad Sci USA. 2009;106(40):17129–17133. doi: 10.1073/pnas.0906956106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wurbel MA, Malissen B, Campbell JJ. Complex regulation of CCR9 at multiple discrete stages of T cell development. Eur J Immunol. 2006;36(1):73–81. doi: 10.1002/eji.200535203. [DOI] [PubMed] [Google Scholar]
- 116.Choi YI, Duke-Cohan JS, Ahmed WB, Handley MA, Mann F, Epstein JA, Clayton LK, Reinherz EL. PlexinD1 glycoprotein controls migration of positively selected thymocytes into the medulla. Immunity. 2008;29(6):888–898. doi: 10.1016/j.immuni.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Annunziato F, Romagnani P, Cosmi L, Beltrame C, Steiner BH, Lazzeri E, Raport CJ, Galli G, Manetti R, Mavilia C, Vanini V, Chantry D, Maggi E, Romagnani S. Macrophage-derived chemokine and EBI1-ligand chemokine attract human thymocytes in different stage of development and are produced by distinct subsets of medullary epithelial cells: possible implications for negative selection. J Immunol. 2000;165(1):238–246. doi: 10.4049/jimmunol.165.1.238. [DOI] [PubMed] [Google Scholar]
- 118.Chantry D, Romagnani P, Raport CJ, Wood CL, Epp A, Romagnani S, Gray PW. Macrophage-derived chemokine is localized to thymic medullary epithelial cells and is a chemoattractant for CD3(+), CD4(+), CD8(low) thymocytes. Blood. 1999;94(6):1890–1898. [PubMed] [Google Scholar]
- 119.Baekkevold ES, Wurbel MA, Kivisakk P, Wain CM, Power CA, Haraldsen G, Campbell JJ. A role for CCR4 in development of mature circulating cutaneous T helper memory cell populations. J Exp Med. 2005;201(7):1045–1051. doi: 10.1084/jem.20041059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wurbel MA, Malissen M, Guy-Grand D, Meffre E, Nussenzweig MC, Richelme M, Carrier A, Malissen B. Mice lacking the CCR9 CC-chemokine receptor show a mild impairment of early T- and B-cell development and a reduction in T-cell receptor gammadelta(+) gut intraepithelial lymphocytes. Blood. 2001;98(9):2626–2632. doi: 10.1182/blood.v98.9.2626. [DOI] [PubMed] [Google Scholar]
- 121.Wurbel MA, Malissen M, Guy-Grand D, Malissen B, Campbell JJ. Impaired accumulation of antigen-specific CD8 lymphocytes in chemokine CCL25-deficient intestinal epithelium and lamina propria. J Immunol. 2007;178(12):7598–7606. doi: 10.4049/jimmunol.178.12.7598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Le Borgne M, Ladi E, Dzhagalov I, Herzmark P, Liao YF, Chakraborty AK, Robey EA. The impact of negative selection on thymocyte migration in the medulla. Nat Immunol. 2009;10(8):823–830. doi: 10.1038/ni.1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ansel KM, Cyster JG. Chemokines in lymphopoiesis and lymphoid organ development. Curr Opin Immunol. 2001;13(2):172–179. doi: 10.1016/s0952-7915(00)00201-6. [DOI] [PubMed] [Google Scholar]
- 124.Derbinski J, Kyewski B. How thymic antigen presenting cells sample the body’s self-antigens. Curr Opin Immunol. 2010;22(5):592–600. doi: 10.1016/j.coi.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 125.Nedjic J, Aichinger M, Emmerich J, Mizushima N, Klein L. Autophagy in thymic epithelium shapes the T-cell repertoire and is essential for tolerance. Nature. 2008;455(7211):396–400. doi: 10.1038/nature07208. [DOI] [PubMed] [Google Scholar]
- 126.Kyewski BA, Fathman CG, Kaplan HS. Intrathymic presentation of circulating non-major histocompatibility complex antigens. Nature. 1984;308(5955):196–199. doi: 10.1038/308196a0. [DOI] [PubMed] [Google Scholar]
- 127.Volkmann A, Zal T, Stockinger B. Antigen-presenting cells in the thymus that can negatively select MHC class II-restricted T cells recognizing a circulating self antigen. J Immunol. 1997;158(2):693–706. [PubMed] [Google Scholar]
- 128.Baba T, Nakamoto Y, Mukaida N. Crucial contribution of thymic Sirp alpha + conventional dendritic cells to central tolerance against blood-borne antigens in a CCR2-dependent manner. J Immunol. 2009;183(5):3053–3063. doi: 10.4049/jimmunol.0900438. [DOI] [PubMed] [Google Scholar]
- 129.Bonasio R, Scimone ML, Schaerli P, Grabie N, Lichtman AH, von Andrian UH. Clonal deletion of thymocytes by circulating dendritic cells homing to the thymus. Nat Immunol. 2006;7(10):1092–1100. doi: 10.1038/ni1385. [DOI] [PubMed] [Google Scholar]
- 130.Tykocinski LO, Sinemus A, Kyewski B. The thymus medulla slowly yields its secrets. Ann N Y Acad Sci. 2008;1143:105–122. doi: 10.1196/annals.1443.018. [DOI] [PubMed] [Google Scholar]
- 131.Aaltonen J, Bjorses P, Sandkuijl L, Perheentupa J, Peltonen L. An autosomal locus causing autoimmune disease: autoimmune polyglandular disease type I assigned to chromosome 21. Nat Genet. 1994;8(1):83–87. doi: 10.1038/ng0994-83. [DOI] [PubMed] [Google Scholar]
- 132.Nagamine K, Peterson P, Scott HS, Kudoh J, Minoshima S, Heino M, Krohn KJ, Lalioti MD, Mullis PE, Antonarakis SE, Kawasaki K, Asakawa S, Ito F, Shimizu N. Positional cloning of the APECED gene. Nat Genet. 1997;17(4):393–398. doi: 10.1038/ng1297-393. [DOI] [PubMed] [Google Scholar]
- 133.Anderson MS, Venanzi ES, Klein L, Chen Z, Berzins SP, Turley SJ, von Boehmer H, Bronson R, Dierich A, Benoist C, Mathis D. Projection of an immunological self-shadow within the thymus by the Aire protein. Science. 2002;298(5597):1395–1401. doi: 10.1126/science.1075958. [DOI] [PubMed] [Google Scholar]
- 134.Abramson J, Giraud M, Benoist C, Mathis D. Aire’s partners in the molecular control of immunological tolerance. Cell. 2010;140(1):123–135. doi: 10.1016/j.cell.2009.12.030. [DOI] [PubMed] [Google Scholar]
- 135.McCaughtry TM, Wilken MS, Hogquist KA. Thymic emigration revisited. J Exp Med. 2007;204(11):2513–2520. doi: 10.1084/jem.20070601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Klein L, Hinterberger M, Wirnsberger G, Kyewski B. Antigen presentation in the thymus for positive selection and central tolerance induction. Nat Rev. 2009;9(12):833–844. doi: 10.1038/nri2669. [DOI] [PubMed] [Google Scholar]
- 137.Ehrlich LIR, Oh DY, Weissman IL, Lewis RS. Differential contribution of chemotaxis and substrate restriction to segregation of immature and mature thymocytes. Immunity. 2009;31(6):986–998. doi: 10.1016/j.immuni.2009.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Anderson MS, Venanzi ES, Chen Z, Berzins SP, Benoist C, Mathis D. The cellular mechanism of Aire control of T cell tolerance. Immunity. 2005;23(2):227–239. doi: 10.1016/j.immuni.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 139.Scollay RG, Butcher EC, Weissman IL. Thymus cell migration. Quantitative aspects of cellular traffic from the thymus to the periphery in mice. Eur J Immunol. 1980;10(3):210–218. doi: 10.1002/eji.1830100310. [DOI] [PubMed] [Google Scholar]
- 140.Hazenberg MD, Borghans JA, de Boer RJ, Miedema F. Thymic output: a bad TREC record. Nat Immunol. 2003;4(2):97–99. doi: 10.1038/ni0203-97. [DOI] [PubMed] [Google Scholar]
- 141.Chaffin KE, Beals CR, Wilkie TM, Forbush KA, Simon MI, Perlmutter RM. Dissection of thymocyte signaling pathways by in vivo expression of pertussis toxin ADP-ribosyltransferase. EMBO J. 1990;9(12):3821–3829. doi: 10.1002/j.1460-2075.1990.tb07600.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Chaffin KE, Perlmutter RM. A pertussis toxin-sensitive process controls thymocyte emigration. Eur J Immunol. 1991;21(10):2565–2573. doi: 10.1002/eji.1830211038. [DOI] [PubMed] [Google Scholar]
- 143.Chiba K. FTY720, a new class of immunomodulator, inhibits lymphocyte egress from secondary lymphoid tissues and thymus by agonistic activity at sphingosine 1-phosphate receptors. Pharmacol Therapeutics. 2005;108(3):308–319. doi: 10.1016/j.pharmthera.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 144.Matloubian M, Lo CG, Cinamon G, Lesneski MJ, Xu Y, Brinkmann V, Allende ML, Proia RL, Cyster JG. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature. 2004;427(6972):355–360. doi: 10.1038/nature02284. [DOI] [PubMed] [Google Scholar]
- 145.Allende ML, Dreier JL, Mandala S, Proia RL. Expression of the sphingosine 1-phosphate receptor, S1P1, on T-cells controls thymic emigration. J Biol Chem. 2004;279(15):15396–15401. doi: 10.1074/jbc.M314291200. [DOI] [PubMed] [Google Scholar]
- 146.Pappu R, Schwab SR, Cornelissen I, Pereira JP, Regard JB, Xu Y, Camerer E, Zheng YW, Huang Y, Cyster JG, Coughlin SR. Promotion of lymphocyte egress into blood and lymph by distinct sources of sphingosine-1-phosphate. Science. 2007;316(5822):295–298. doi: 10.1126/science.1139221. [DOI] [PubMed] [Google Scholar]
- 147.Vogel P, Donoviel MS, Read R, Hansen GM, Hazlewood J, Anderson SJ, Sun W, Swaffield J, Oravecz T. Incomplete inhibition of sphingosine 1-phosphate lyase modulates immune system function yet prevents early lethality and non-lymphoid lesions. PloS one. 2009;4(1):e4112. doi: 10.1371/journal.pone.0004112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Zachariah MA, Cyster JG. Neural crest-derived pericytes promote egress of mature thymocytes at the corticomedullary junction. Science. 2010;328(5982):1129–1135. doi: 10.1126/science.1188222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Shiow LR, Rosen DB, Brdickova N, Xu Y, An J, Lanier LL, Cyster JG, Matloubian M. CD69 acts downstream of interferon-alpha/beta to inhibit S1P1 and lymphocyte egress from lymphoid organs. Nature. 2006;440(7083):540–544. doi: 10.1038/nature04606. [DOI] [PubMed] [Google Scholar]
- 150.Carlson CM, Endrizzi BT, Wu J, Ding X, Weinreich MA, Walsh ER, Wani MA, Lingrel JB, Hogquist KA, Jameson SC. Kruppel-like factor 2 regulates thymocyte and T-cell migration. Nature. 2006;442(7100):299–302. doi: 10.1038/nature04882. [DOI] [PubMed] [Google Scholar]
- 151.Fabre S, Carrette F, Chen J, Lang V, Semichon M, Denoyelle C, Lazar V, Cagnard N, Dubart-Kupperschmitt A, Mangeney M, Fruman DA, Bismuth G. FOXO1 regulates L-Selectin and a network of human T cell homing molecules downstream of phosphatidylinositol 3-kinase. J Immunol. 2008;181(5):2980–2989. doi: 10.4049/jimmunol.181.5.2980. [DOI] [PubMed] [Google Scholar]
- 152.Barbee SD, Alberola-Ila J. Phosphatidylinositol 3-kinase regulates thymic exit. J Immunol. 2005;174(3):1230–1238. doi: 10.4049/jimmunol.174.3.1230. [DOI] [PubMed] [Google Scholar]
- 153.Ueno T, Hara K, Willis MS, Malin MA, Hopken UE, Gray DH, Matsushima K, Lipp M, Springer TA, Boyd RL, Yoshie O, Takahama Y. Role for CCR7 ligands in the emigration of newly generated T lymphocytes from the neonatal thymus. Immunity. 2002;16(2):205–218. doi: 10.1016/s1074-7613(02)00267-4. [DOI] [PubMed] [Google Scholar]
- 154.Vianello F, Kraft P, Mok YT, Hart WK, White N, Poznansky MC. A CXCR4-dependent chemorepellent signal contributes to the emigration of mature single-positive CD4 cells from the fetal thymus. J Immunol. 2005;175(8):5115–5125. doi: 10.4049/jimmunol.175.8.5115. [DOI] [PubMed] [Google Scholar]
- 155.Srivatsan S, Peng SL. Cutting edge: Foxj1 protects against autoimmunity and inhibits thymocyte egress. J Immunol. 2005;175(12):7805–7809. doi: 10.4049/jimmunol.175.12.7805. [DOI] [PubMed] [Google Scholar]
- 156.Shiow LR, Roadcap DW, Paris K, Watson SR, Grigorova IL, Lebet T, An J, Xu Y, Jenne CN, Foger N, Sorensen RU, Goodnow CC, Bear JE, Puck JM, Cyster JG. The actin regulator coronin 1A is mutant in a thymic egress-deficient mouse strain and in a patient with severe combined immunodeficiency. Nat Immunol. 2008;9(11):1307–1315. doi: 10.1038/ni.1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Sakata D, Taniguchi H, Yasuda S, Adachi-Morishima A, Hamazaki Y, Nakayama R, Miki T, Minato N, Narumiya S. Impaired T lymphocyte trafficking in mice deficient in an actin-nucleating protein, mDia1. J Exp Med. 2007;204(9):2031–2038. doi: 10.1084/jem.20062647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Morley SC, Wang C, Lo WL, Lio CW, Zinselmeyer BH, Miller MJ, Brown EJ, Allen PM. The actin-bundling protein L-plastin dissociates CCR7 proximal signaling from CCR7-induced motility. J Immunol. 2010;184(7):3628–3638. doi: 10.4049/jimmunol.0903851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Katagiri K, Ohnishi N, Kabashima K, Iyoda T, Takeda N, Shinkai Y, Inaba K, Kinashi T. Crucial functions of the Rap1 effector molecule RAPL in lymphocyte and dendritic cell trafficking. Nat Immunol. 2004;5(10):1045–1051. doi: 10.1038/ni1111. [DOI] [PubMed] [Google Scholar]
- 160.Dong Y, Du X, Ye J, Han M, Xu T, Zhuang Y, Tao W. A cell-intrinsic role for Mst1 in regulating thymocyte egress. J Immunol. 2009;183(6):3865–3872. doi: 10.4049/jimmunol.0900678. [DOI] [PubMed] [Google Scholar]
- 161.Kato S. Thymic microvascular system. Microsc Res Tech. 1997;38(3):287–299. doi: 10.1002/(SICI)1097-0029(19970801)38:3<287::AID-JEMT9>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 162.Griffith AV, Fallahi M, Nakase H, Gosink M, Young B, Petrie HT. Spatial mapping of thymic stromal microenvironments reveals unique features influencing T lymphoid differentiation. Immunity. 2009;31(6):999–1009. doi: 10.1016/j.immuni.2009.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Gill J, Malin M, Sutherland J, Gray D, Hollander G, Boyd R. Thymic generation and regeneration. Immunol Rev. 2003;195:28–50. doi: 10.1034/j.1600-065x.2003.00077.x. [DOI] [PubMed] [Google Scholar]
- 164.van den Brink MRM, Alpdogan O, Boyd RL. Strategies to enhance T-cell reconstitution in immunocompromised patients. Nat Rev Immunol. 2004;4(11):856–867. doi: 10.1038/nri1484. [DOI] [PubMed] [Google Scholar]
- 165.Zlotoff DA, Bhandoola A. Hematopoietic progenitor migration to the adult thymus. Ann NY Acad Sci. 2011;1217(1):122–138. doi: 10.1111/j.1749-6632.2010.05881.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Mandala S, Hajdu R, Bergstrom J, Quackenbush E, Xie J, Milligan J, Thornton R, Shei G-J, Card D, Keohane C, Rosenbach M, Hale J, Lynch CL, Rupprecht K, Parsons W, Rosen H. Alteration of lymphocyte trafficking by sphingosine-1-phosphate receptor agonists. Science. 2002;296(5566):346–349. doi: 10.1126/science.1070238. [DOI] [PubMed] [Google Scholar]
- 167.Liu G, Yang K, Burns S, Shrestha S, Chi H. The S1P(1)-mTOR axis directs the reciprocal differentiation of T(H)1 and T(reg) cells. Nat Immunol. 2010;11(11):1047–1056. doi: 10.1038/ni.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Brinkmann V, Billich A, Baumruker T, Heining P, Schmouder R, Francis G, Aradhye S, Burtin P. Fingolimod (FTY720): discovery and development of an oral drug to treat multiple sclerosis. Nat Rev Drug Discov. 2010;9(11):883–897. doi: 10.1038/nrd3248. [DOI] [PubMed] [Google Scholar]
- 169.Calderon L, Boehm T (2011) Three chemokine receptors cooperatively regulate homing of hematopoietic progenitors to the embryonic mouse thymus. Proc Natl Acad Sci U S A 108(18):7517–7522. doi:1016428108 [pii] 10.1073/pnas.1016428108 [DOI] [PMC free article] [PubMed]
- 170.Janas ML, Varano G, Gudmundsson K, Noda M, Nagasawa T, Turner M. Thymic development beyond beta-selection requires phosphatidylinositol 3-kinase activation by CXCR4. J Exp Med. 2010;207(1):247–261. doi: 10.1084/jem.20091430. [DOI] [PMC free article] [PubMed] [Google Scholar]