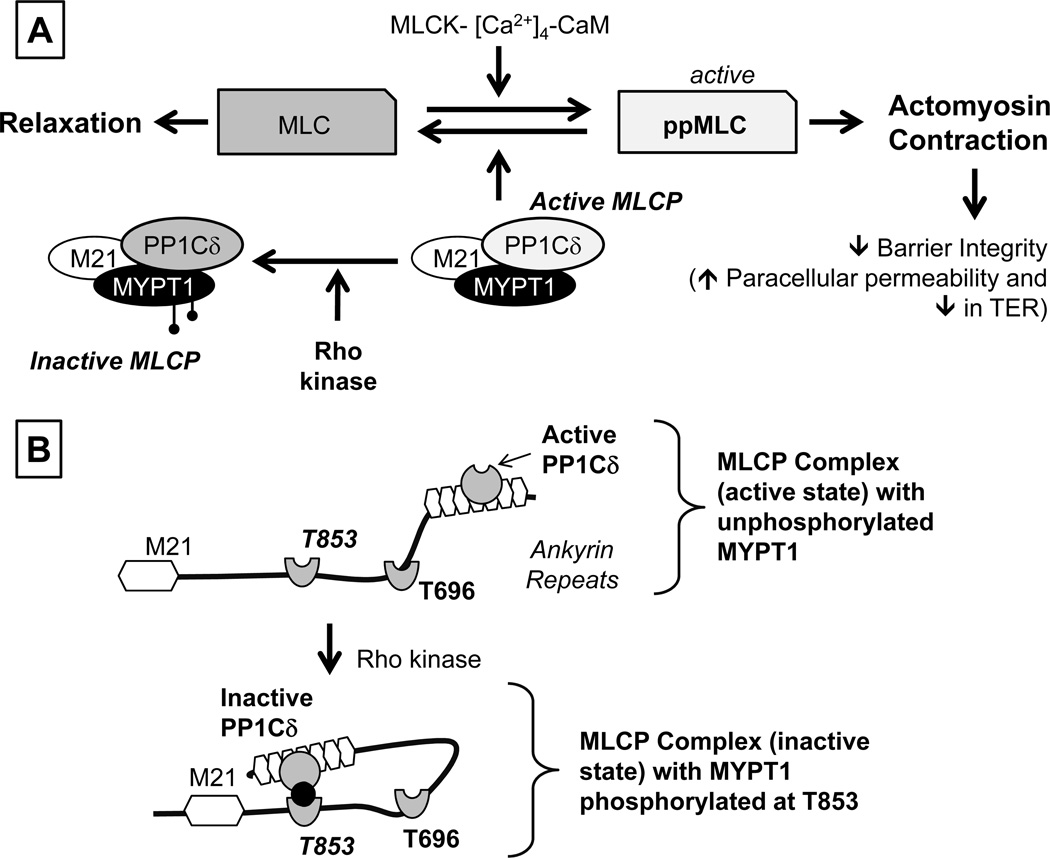
Figure 2. Overview of signaling pathways underlying actomyosin contraction.
Panel A: Myosin light chain (MLC) phosphorylation is catalyzed by Ca2+-Calmodulin-dependent MLC kinase (MLCK). The expression of both endothelial and smooth muscle isoforms are seen in the corneal endothelium. MLC phosphorylation promotes actomyosin interaction, leading to increased cellular contractility. MLCK activity is opposed by MLC phosphatase (MLCP), which catalyzes dephosphorylation of ppMLC. MLCP is a heterotrimeric complex consisting of MYPT1 (a regulatory subunit), PP1Cδ (protein phosphatase 1; catalytic subunit δ-isoform), and M21 (function unknown). Rho kinase, a RhoA effector, phosphorylates MYPT1, thereby inhibiting PP1Cδ. Panel B: Autoinhibition of MLCP: The activity of PP1Cδ is inhibited by ROCK through phosphorylation of MYPT1 at T853 or T696. Specifically, the substrate site of PP1Cδ is accessible when neither T696 nor T853 is unphosphorylated. However, when MYPT1 is phosphorylated at T696 or T853 (phosphorylation of T853 is shown here), the phosphorylated residues interact with the active site of PP1Cδ and suppress the phosphatase activity. Adapted from our previous report (Ramachandran et al., 2011).