Fig. 2.
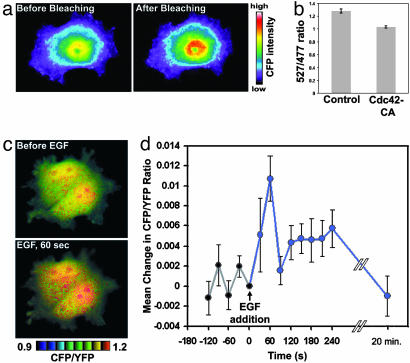
Stinger can be used to visualize regulated N-WASP activity changes in living cells. (a) Stinger undergoes FRET in vivo. To verify the existence of intramolecular FRET of Stinger in live HEK-293 cells, CFP emission was visualized before and after YFP photobleaching. CFP emission increased after YFP photobleaching, confirming Stinger FRET. (b) Constitutively active Cdc42 causes a reduction of Stinger FRET efficiency in live cells. Live HEK-293 cells expressing either Stinger alone or Stinger plus Cdc42-CA were used for dual-emission ratio imaging. The mean ratio of YFP/CFP emission during CFP excitation was then determined. Ten random fields of view were imaged by using a ×10 objective for each group, each containing 50–100 cells. Error bars represent SEM. (c) EGF causes a transient activation of N-WASP in COS-7 cells. Cells expressing Stinger were serum-starved overnight and imaged before and 1 min after addition of 50 ng/ml EGF. FRET efficiency is represented by intensity-modulated display (IMD) coloring, in which CFP/YFP ratio values determine the color of each pixel and intensity values determine the brightness of each pixel. Higher ratio values of CFP/YFP (warm colors) indicate a reduction of FRET efficiency and represent increased N-WASP activity. (d) Quantification of N-WASP activation after EGF addition. Cells expressing Stinger were serum-starved overnight and then exposed to 50 ng/ml EGF at time 0. Dual-emission ratio imaging of individual cells was conducted at 1-min intervals before and after stimulation, and the mean CFP/YFP ratio for each cell was then determined. The mean changes in CFP/YFP (compared with time 0) of 19 cells are shown. EGF caused a statistically significant increase in N-WASP activity 1 min after addition (paired t test, P < 0.005, n = 19). Error bars represent SEM.