Abstract
The Ras effector NORE1 is frequently silenced in primary adenocarcinomas, although the significance of this silencing for tumorigenesis is unclear. Here we show that NORE1 induces polyubiquitination and proteasomal degradation of oncoprotein HIPK1 by facilitating its interaction with the Mdm2 E3 ubiquitin ligase. Endogenous HIPK1 is stabilized in Nore1-deficient mouse embryonic fibroblasts, and depletion of HIPK1 in NORE1-silenced lung adenocarcinoma cells inhibits anchorage-independent cell growth and tumour formation in nude mice. These findings indicate that the control of HIPK1 stability by Mdm2–NORE1 has a major effect on cell behaviour, and epigenetic inactivation of NORE1 enables adenocarcinoma formation in vivo through HIPK1 stabilization.
Keywords: Novel Ras effector 1, RASSF5, homeodomain-interacting protein kinase 1, oncogene; tumorigenesis
Introduction
NORE1 (Novel Ras effector 1, also called RASSF5) is the founding member of the RASSF gene family (van der Weyden & Adams, 2007; Richter et al, 2009). Nore1 is a proapoptotic effector of Ras, which binds to Mst1, a protein kinase implicated in apoptosis (Khokhlatchev et al, 2002). Nore1-null mice were resistant to tumour necrosis factor-α-induced apoptosis. In addition, loss of Nore1 resulted in spontaneous immortalization of mouse embryonic fibroblasts (MEFs), and Nore1-null immortalized MEFs were fully transformed by K-RasG12V (Park et al, 2010). Methylation of the NORE1A promoter has been reported in various tumour types including lung, kidney and breast tumour cell lines and primary tumours (Richter et al, 2009). As the loss of NORE1 expression has been linked to tumour formation, identification of the downstream effector(s) of Nore1 is crucial to understand the mechanism by which tumour formation is induced by loss of NORE1 expression.
Homeodomain-interacting protein kinase 1 (HIPK1) was identified as an NK class homeodomain protein-interacting protein and was shown to be involved in the transcriptional regulation of target genes as a co-repressor or coactivator in a context-dependent manner (Kim et al, 1998). HIPK1 participates in haematopoietic cell lineage differentiation and blood vessel formation (Aikawa et al, 2006). HIPK1 knockout (KO) mice appear grossly normal with no obvious effect on viability (Aikawa et al, 2006; Isono et al, 2006), but HIPK1 knockout mice are resistant to drug-induced skin tumorigenesis, implying an oncogenic role of HIPK1 (Kondo et al, 2003). Consistent with this potential oncogenic role, HIPK1 was found to be overexpressed in E1A/Ras-transformed MEFs and in a variety of breast cancer cell lines (Kondo et al, 2003). However, the mechanism of HIPK1 involvement in tumorigenesis and the physiological relevance of HIPK1 overexpression in cancer cell lines are as yet unclear.
Here we show that Nore1 facilitates Mdm2-mediated proteasomal degradation of HIPK1, and that HIPK1 is stabilized in Nore1-null immortalized MEFs. The tumorigenic activity of A549 lung cancer cells, in which NORE1A was silenced because of NORE1A promoter methylation, was reversed on suppression of HIPK1 expression in a soft agar assay and xenograft nude mice model. These results provide new insight into the tumorigenic mechanism of NORE1A promoter methylation, where suppression of NORE1A expression leads to stabilization of the HIPK1 oncoprotein, resulting in tumour formation in vivo.
Results And Discussion
Nore1 induces degradation of HIPK1
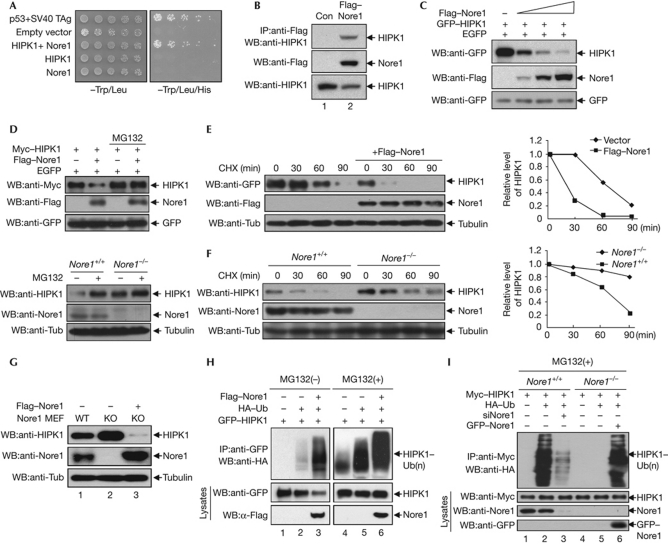
To identify proteins that bind to and potentially regulate the function of HIPK1, a yeast two-hybrid screen of a mouse embryonic complementary DNA library was performed using the HIPK1 C terminus (amino acids 520–1209) as bait. Nore1 was identified as a HIPK1-interacting protein through this screen (Fig 1A). The specificity of HIPK1 binding to Nore1 was confirmed by a coimmunoprecipitation assay in mammalian cells (Fig 1B). Endogenous HIPK1 co-precipitated with endogenous Nore1, and coexpression of green fluorescent protein (GFP)–HIPK1 and Flag–Nore1 resulted in the colocalization of the two proteins to the discrete dot structures in the nucleus (supplementary Fig S1 online). In addition, glutathione S-transferase (GST) pull-down analysis showed that Nore1 physically interacts with HIPK1 (supplementary Fig S1B online). During the coimmunoprecipitation experiments, we found unexpectedly that the level of HIPK1 was reduced by Nore1 expression (Fig 1B, lane 2, bottom panel). Subsequently, a series of experiments was conducted to examine whether HIPK1 expression is modulated by Nore1. Expression of increasing amounts of Nore1 in COS7 cells reduced the HIPK1 level in a dose-dependent manner (Fig 1C). The decrease in HIPK1 levels in the presence of Nore1 was blocked by the administration of the proteasome inhibitor MG132 (Fig 1D). To confirm whether Nore1 causes a decrease in HIPK1, the stability of HIPK1 was determined in the presence of cycloheximide. Coexpression of Flag–Nore1 resulted in a markedly reduced half-life of GFP–HIPK1 protein (<30 min; Fig 1E). In addition, the stability of endogenous HIPK1 in Nore1-KO MEFs was higher than in wild-type MEF cells (Fig 1F). When Nore1 was reintroduced into Nore1-KO MEFs, the level of endogenous HIPK1 decreased, showing that Nore1 destabilizes HIPK1 protein (Fig 1G). Consistently, western blot analysis showed that polyubiquitination of HIPK1 increased on coexpression with Nore1, and MG132 treatment caused an overall accumulation of polyubiquitinated HIPK1 (Fig 1H). We also found that HIPK1 was polyubiquitinated in Nore1-KO MEFs only when Nore1 was reintroduced, verifying that polyubiquitination of HIPK1 depends on Nore1 (Fig 1I, lane 6). Collectively, these results showed that Nore1 regulates the stability of HIPK1 by inducing polyubiquitination and proteasome-dependent degradation of HIPK1.
Figure 1.
Nore1-mediated polyubiquitination and degradation of HIPK1 in mammalian cells. (A) The Nore1 (Novel Ras effector 1)-encoding yeast clone and GAL–DBD–HIPK1 plasmid were cotransformed into the yeast AH109 strain. Cells were serially diluted and plated on synthetic dropout plates lacking either Trp/Leu or Trp/Leu/His. (B) HEK293 cells expressing Flag–Nore1 were immunoprecipitated (IP) with anti-Flag antibody, followed by western blotting (WB) using anti-HIPK1 antibody. (C) Increasing amounts of Flag–Nore1 expression plasmids were co-transfected into COS7 cells, followed by western blotting using anti-GFP or anti-Flag antibodies. (D) Myc–HIPK1 plasmids were transfected into COS7 cells in the presence or absence of Flag–Nore1 plasmids, and cells were treated with 5 μM MG132 for 12 h. GFP expression was measured as a transfection control (upper panels). Nore1 wild-type (WT) mouse embryo fibroblasts (MEFs) and Nore1-KO MEFs were treated with MG132 for 12 h, and HIPK1 levels were determined using anti-HIPK1 antibody (lower panels) (E) Analysis of GFP–HIPK1 half-life in the presence or absence of Flag–Nore1. The relative levels of HIPK1 with or without Nore1 expression are quantified on the right panel. (F) HIPK1 levels were increased in Nore1-KO MEF cells compared with wild-type cells, as determined by western blotting using anti-HIPK1 antibody. (G) Introduction of Flag–Nore1 into Nore1-KO MEF cells causes HIPK1 protein levels to decrease. (H) The GFP–HIPK1 expression plasmid was transfected into COS7 cells in combination with HA–ubiquitin and the Flag–Nore1 expression plasmid. Half of the transfected cells were treated with MG132 for 12 h before analysis. (I) Nore1 causes polyubiquitination of HIPK1. Cell lysates were prepared from cells expressing the indicated proteins, and were immunoprecipitated with Myc antibody, followed by western blotting using anti-HA antibody to determine polyubiquitination of HIPK1. CHX, cycloheximide; EGFP, enhanced green fluorescent protein; GFP, green fluorescent protein; HA, haemagglutinin; KO, knockout; Si, short interfering; TAg, T antigen; Tub, tubulin; Ub, ubiquitin.
Nore1 degrades HIPK1 through the E3 ligase Mdm2
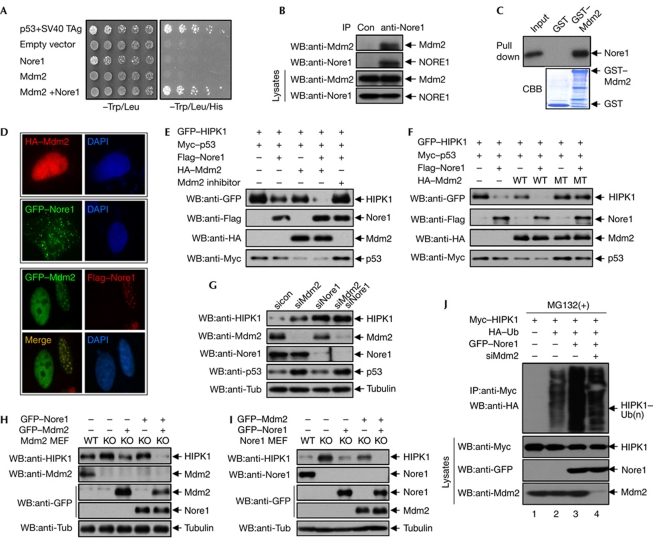
Nore1 has high similarity in amino acids and domain organization with RASSF1. RASSF1 interacts with the E3 ubiquitin ligase Mdm2 via its C1-type zinc-finger motif, which is well conserved in Nore1 (Song et al, 2008). We therefore proposed that Nore1-bound Mdm2 might cause the polyubiquitination and degradation of HIPK1. In a yeast two-hybrid assay, an Mdm2–GAL4 DNA-binding domain fusion protein interacted with a Nore1–GAL4 activation domain fusion protein (Fig 2A). In coimmunoprecipitation assays in mammalian cells, endogenous Mdm2 was observed to be bound to endogenous Nore1 (Fig 2B). In GST pull-down assay, Nore1 bound to GST–Mdm2 but not to GST (Fig 2C). These results indicate that Nore1 interacts with Mdm2 in vitro and in vivo. Consistently, coexpression with Nore1 caused Mdm2 to colocalize with Nore1 in U2OS cells (Fig 2D). Next, we examined the role of Mdm2 in Nore1-mediated HIPK1 degradation. Nore1-induced degradation of HIPK1 was further enhanced with Mdm2 coexpression. However, when the transfected cells were treated with an Mdm2 inhibitor that blocks E3 ligase activity, Nore1-induced degradation of HIPK1 was inhibited (Fig 2E). Expression of Mdm2 alone resulted in only a slight decrease in HIPK1. Coexpression of Mdm2 and Nore1 resulted in a greater decrease in HIPK1, which was not observed when the catalytically inactive C462A Mdm2 mutant was coexpressed (Fig 2F). Next, we examined Mdm2 function on HIPK1 stability under physiological conditions. Endogenous HIPK1 levels increased when endogenous Mdm2 or Nore1 was knocked down in U2OS cells using short interfering RNAs (siRNAs). Knockdown of both Mdm2 and Nore1 resulted in an increase in endogenous HIPK1 to a level similar to the Nore1 knockdown cells, indicating that Mdm2 and Nore1 affect HIPK1 stability in a non-additive manner (Fig 2G). This result indicates that Mdm2 and Nore1 exert their functions on the same pathway. In addition, whereas p53/Mdm2-double KO MEFs showed an increase in HIPK1 stability compared with p53-KO MEFs, reintroduction of Mdm2 into Mdm2-KO MEFs resulted in HIPK1 degradation (Fig 2H). Furthermore, expression of both Nore1 and Mdm2 resulted in lower levels of HIPK1 than either expression of Mdm2 alone into Mdm2-KO MEFs or expression of Nore1 alone into Nore1-KO MEFs (Fig 2H,I). Using Mdm2 siRNA, we tested whether Mdm2 is involved in Nore1-mediated polyubiquitination of HIPK1. Nore1-induced enhancement of HIPK1 polyubiquitination was reduced when Mdm2 was knocked down (Fig 2J). These results show that Nore1 induces the degradation of HIPK1 through the E3 ubiquitin ligase activity of Mdm2.
Figure 2.
Nore1 degrades HIPK1 through the E3 ligase activity of Mdm2. (A) Yeast AH109 cells were transformed with expression plasmids encoding Mdm2–GAL4 DNA-binding domain and Nore1 (Novel Ras effector 1)–GAL4 activation domain. Cells were serially diluted and plated on synthetic dropout plates lacking either Trp/Leu or Trp/Leu/His. (B) Coimmunoprecipitation of Mdm2 with Nore1. U2OS cells immunoprecipitated with anti-Nore1 antibody, followed by western blotting using anti-Mdm2 antibody. (C) In vitro translated Myc–Nore1 and affinity-purified glutathione S-transferase (GST)–Mdm2 were subjected to an in vitro binding assay. (D) Localization of either HA–Mdm2 alone or GFP–Nore1 alone (upper panels). Colocalization of GFP–Mdm2 with Flag–Nore1 in U2OS cells (bottom panels). (E) COS7 cells were transfected with the indicated combinations of GFP–HIPK1, Flag–Nore1, Myc–p53 or HA–Mdm2, and subjected to treatment with an Mdm2 inhibitor. Whole-cell lysates were subjected to western blotting. (F) COS7 cells were transfected with GFP–HIPK1, Myc–p53 and Flag–Nore1 plasmids in combination with either wild-type (WT) or catalytically inactive (C462A) HA–Mdm2. Whole-cell lysates were subjected to western blotting. (G) U2OS cells were transfected with Mdm2 and Nore1 siRNA as indicated, followed by western blotting using anti-Mdm2, anti-HIPK1 and anti-Nore1 antibodies. p53 level was determined as a positive control for Mdm2 knockdown. (H) p53/Mdm2 double-KO mouse embryo fibroblasts (MEFs) were transfected with Nore1 and Mdm2 expression plasmids as indicated, and cell lysates of p53-KO (Mdm2 WT) and p53/Mdm2 double-KO MEFs (Mdm2 KO) were subjected to western blotting. (I) Nore1-KO MEFs were transfected with Nore1 and Mdm2 expression plasmids as indicated, and cell lysates were subjected to western blotting. (J) U2OS cells were transfected with Myc–HIPK1 in combination with HA–ubiquitin, GFP–Nore1 and Mdm2-targeting short interfering RNA (siRNA), and cell lysates were immunoprecipitated with Myc antibody, followed by western blotting using anti-HA antibody. CBB, Coomassie brilliant blue; DAPI, 4,6-diamidino-2-phenylindole; GFP, green fluorescent protein; HA, haemagglutinin; IP, immunoprecipitation; KO, knockout; MEF, mouse embryonic fibroblast; sicon, control siRNA; TAg, T antigen; Tub, tubulin; Ub, ubiquitin; WB, western blotting.
Nore1 enhances interaction between HIPK1 and Mdm2
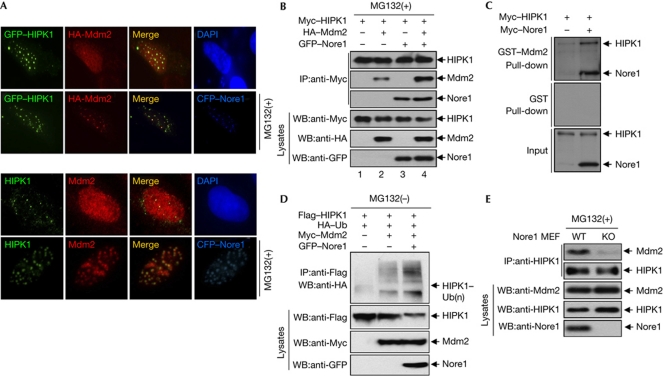
As we had observed that Nore1 colocalized with either HIPK1 or Mdm2 in nuclear dots (supplementary Fig S1 online; Fig 2D), we tested whether HIPK1 could also affect Mdm2 localization. When GFP–HIPK1 and haemagglutinin (HA)–Mdm2 plasmids were co-transfected into U2OS cells, Mdm2 expression remained diffuse, indicating that HIPK1 by itself was not sufficient to induce nuclear dot localization of Mdm2 (Fig 3A, first row of top panels). However, coexpression of cyan fluorescent protein (CFP)–Nore1 along with both GFP–HIPK1 and HA–Mdm2 in the presence of MG132 resulted in colocalization of all three proteins to discrete nuclear dot structures (Fig 3A, second row of top panels). This result indicates that Nore1 recruits Mdm2 and HIPK1 on the nuclear dots for HIPK1 degradation. To determine whether the Nore1-induced nuclear dots are associated with HIPK1 degradation, the number and size of the nuclear dots containing endogenous HIPK1 and Mdm2 were determined in the presence of CFP–Nore1. The Nore1-induced nuclear dots harbouring endogenous HIPK1 and Mdm2 were stabilized by MG132 treatment (Fig 3A, bottom panels), indicating association of HIPK1 degradation with nuclear dots. We subsequently tested whether Nore1 could affect the interaction between HIPK1 and Mdm2 by coimmunoprecipitation and GST pull-down experiments. Myc–HIPK1 was observed to bind to HA–Mdm2 in mammalian cells, and the amount of bound HA–Mdm2 increased when GFP–Nore1 was coexpressed (Fig 3B, lane 4). In addition, a GST pull-down assay revealed that the affinity of HIPK1 binding to GST–Mdm2 was significantly increased in the presence of Nore1 (Fig 3C). Consistently, Mdm2-mediated HIPK1 polyubiquitination levels were further increased by Nore1 coexpression (Fig 3D). Using Nore1-KO MEFs and antibodies against endogenous HIPK1 and Mdm2, we observed that the amount of Mdm2 protein binding to HIPK1 was greatly decreased in Nore1-KO MEFs (Fig 3E). These results indicate that Nore1 might function as a scaffold protein that enhances association between HIPK1 and Mdm2.
Figure 3.
Nore1 enhances interaction between HIPK1 and Mdm2. (A) U2OS cells were transfected with expression plasmids encoding GFP–HIPK1, HA–Mdm2 and CFP–Nore1 (Novel Ras effector 1) as indicated, and their localizations were determined after treatment of cells with MG132 (upper panels). U2OS cells were transfected with CFP–Nore1 expression plasmid, and the localization of endogenous HIPK1 and Mdm2 was determined by immunostaining using anti-HIPK1 and anti-Mdm2 antibodies in the presence of MG132 (bottom panels). (B) Coimmunoprecipitation assay showing that HIPK1-bound Mdm2 levels increase in the presence of Nore1. The transfected cells were treated with MG132 for 12 h, and whole-cell lysates were immunoprecipitated with Myc antibody, followed by western blotting using anti-HA and anti-GFP antibodies. (C) Glutathione S-transferase (GST) pull-down assay showing that Mdm2-bound HIPK1 levels increase in the presence of Nore1. (D) HeLa cells were transfected with plasmids encoding Flag–HIPK1 and HA–ubiquitin in combination with Myc–Mdm2 and GFP–Nore1. Cell lysates were immunoprecipitated with an anti-Flag antibody, followed by western blotting. (E) Levels of endogenous Mdm2 associated with endogenous HIPK1 are decreased in Nore1-KO MEF cells. Nore1-WT and Nore1-KO MEF cells were treated with MG132 for 12 h before harvesting. Cells were lysed and immunoprecipitated with anti-HIPK1 antibody, and associated Mdm2 was detected by western blotting. CFP, cyan fluorescent protein; DAPI, 4,6-diamidino-2-phenylindole; GFP, green fluorescent protein; HA, haemagglutinin; IP, immunoprecipitation; KO, knockout; Ub, ubiquitination; WB, western blotting.
HIPK1 knockdown reduces tumorigenicity of A549 cells
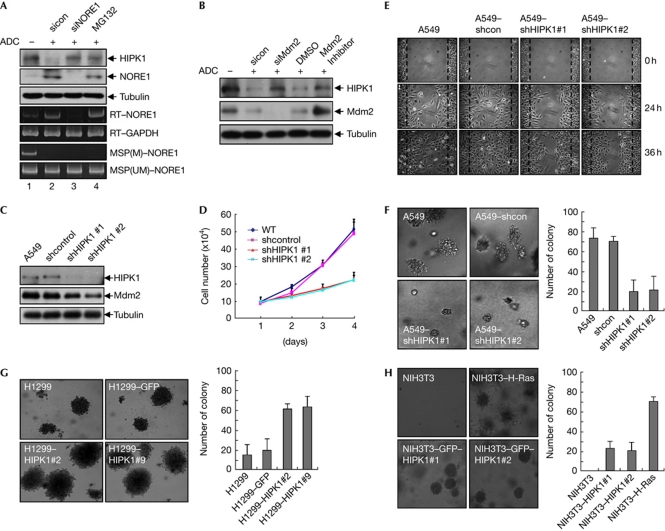
Hypermethylation of the NORE1A promoter has been associated with tumorigenesis in tissues such as the lung, kidney and liver (Richter et al, 2009). To extend our understanding of NORE1 regulation of HIPK1 in cancer cell lines, we used the A549 lung carcinoma cell in which NORE1A is silenced by hypermethylation of its promoter. NORE1 siRNA treatment of 5-Aza-2-deoxycytidine-exposed A549 cells resulted in recovery of HIPK1 levels to the levels of MG132-treated cells (Fig 4A), suggesting that HIPK1 is stabilized by methylation-mediated suppression of NORE1A expression in A549 cells. Consistently, either knockdown of Mdm2 expression or treatment of Mdm2 inhibitor resulted in the stabilization of endogenous HIPK1 in A549 cells (Fig 4B). To explore whether the tumorigenic potential of A549 cells can be influenced by changes in HIPK1 levels, we established A549 cell lines stably expressing HIPK1 short hairpin (sh) RNA (Fig 4C). We then determined the effect of HIPK1 knockdown on proliferation, cell migration and anchorage-independent growth of A549 cells. The rate of cell growth in HIPK1 knockdown cell lines was markedly reduced when compared with parental and control A549 cells (shcontrol; Fig 4D). A wound healing assay also revealed that knockdown of HIPK1 inhibited the migration of A549 cells (Fig 4E). In addition, depletion of HIPK1 reduced the number and size of colonies produced in soft agar assay (Fig 4F). These results were not due to the downregulation of Mdm2, a transcriptional target of HIPK1, because p53 was not elevated in HIPK1 knockdown cells (supplementary Fig S2 online). Increased cell colony formation is indicative of higher potential for cell transformation and thus greater tumorigenic risk. To validate in vivo tumour-forming capabilities, A549-parental cells and A549-derived HIPK1 knockdown cell lines were subcutaneously injected into athymic nude mice. Consistent with the results of the soft agar assay, whereas both A549-parental and A549-shcontrol cells produced large and aggressive tumours, the two independent A549–shHIPK1 cell lines generated smaller ones (supplementary Fig S3 online). These results indicate that the tumorigenic activity of NORE1-silenced A549 adenocarcinoma cells is likely to be due to upregulation of HIPK1 levels. To determine functional relevance between HIPK1 expression levels and its tumorigenic activity, H1299 cell lines stably expressing either GFP or GFP–HIPK1 were established (supplementary Fig S4 online). The production of colonies with H1299 cells expressing GFP–HIPK1 increased fourfold in soft agar assays (Fig 4G), suggesting that overexpression of HIPK1 might be associated with tumorigenic activity. Furthermore, NIH3T3 cells stably expressing GFP–HIPK1 formed anchorage-independent colonies in a soft agar assay, whereas parental NIH3T3 cells did not (Fig 4H; supplementary Fig S4 online), indicating oncogenic function of HIPK1 in normal cells. To establish the clinical relevance of our findings, we determined the methylation status of the NORE1A promoter in 25 human lung cancer patient samples and normal tissue adjacent to cancers using the methylation-specific polymerase chain reaction. Among the 25 samples, six primary tumours were found to have methylated NORE1A promoters, but not in their normal tissue counterparts. Western blotting of the six primary tumours revealed that HIPK1 levels were highly elevated in these tumours compared with their normal counterparts. Elevated expression of HIPK1 in the tumours was further confirmed by immunostaining the tumour tissues with anti-HIPK1 antibodies (supplementary Fig S5 online). These results indicate that inhibition of NORE1A expression via methylation of its promoter and the resulting stabilization of HIPK1 might contribute to lung cancer pathogenesis.
Figure 4.
HIPK1 knockdown reduces tumorigenic activity of A549 lung carcinoma cells. (A) NORE1 (Novel Ras effector 1) short interfering RNA (siRNA) treatment of 5-ADC-exposed A549 cells resulted in recovery of HIPK1 levels. A549 lung adenocarcinoma cells were exposed to 5-ADC for 4 days and subjected to NORE1 siRNA or MG132 treatment. Transcription and methylation status of the NORE1A gene was verified by reverse transcription polymerase chain reaction (RT–PCR) with NORE1-specific primers or methylation-specific PCR using genomic DNA, respectively. (B) Knockdown of Mdm2 expression resulted in the stabilization of endogenous HIPK1 in 5-ADC-exposed A549 cells. (C) A549-derived cell lines expressing HIPK1 short hairpin RNA (shRNA) were established. Parental A549 cells and A549-derived cell lines (shcontrol, shHIPK1#1 and #2) were lysed and subjected to western blotting. (D) Equal numbers (5 × 104 cells) of A549-parental cells and A549-derived knockdown cells were plated on a 60-mm dish, and the numbers of cells were counted daily. The graph depicts the mean±s.e.m. of triplicate experiments. (E) A wound healing assay to assess migration ability of control and HIPK1 shRNA cell lines. (F) HIPK1 knockdown reduced the number and size of colonies produced in a soft agar assay. The graph depicts the mean number of colonies larger than 100 μm in diameter, and the error bars represent the s.e.m. of triplicate results. (G) Overexpression of HIPK1 in H1299 cells increased the number and size of colonies produced in a soft agar assay. The graph depicts the mean number of colonies larger than 150 μm in diameter and the error bars represent the s.e.m. of triplicate results. (H) Two different NIH3T3 cells stably expressing GFP–HIPK1 formed anchorage-independent colonies in a soft agar assay. H-Ras-activated mutant (Q61L) was used as a positive control. The graph depicts the mean number of colonies larger than 150 μm in diameter and the error bars represent the s.e.m. of triplicate results. ADC, 5-Aza-2-deoxycytidine; DMSO, dimethylsulphhoxide; GFP, green fluorescent protein; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; MSP, methylation-specific PCR; sicon, control siRNA; WT, wild type.
The oncogenic potential of HIPK1 was initially proposed in a HIPK1-KO mouse model in which tumour formation was markedly reduced in HIPK1-KO mice after topical exposure to a chemical carcinogen (Kondo et al, 2003). In addition, the HIPK1 gene was highly expressed in human breast cancer cell lines and oncogenically transformed MEFs (Kondo et al, 2003). Here we have provided evidence that HIPK1 is able to transform normal cells such as NIH3T3 cells, and epigenetic inactivation of NORE1A by promoter hypermethylation can cause upregulation of HIPK1, probably contributing to tumorigenesis. We therefore propose that HIPK1 stabilization might have a main causative role in the tumorigenesis of lung cancer mediated by NORE1A silencing.
Methods
Cell culture and treatment. COS7 and HeLa cells were grown in Dulbecco's modified Eagle's medium, supplemented with 10% fetal bovine serum (FBS). U2OS and A549 cells were grown in RPMI1640 medium, supplemented with 10% FBS. Primary Nore1+/+ and Nore1−/− MEF cells were previously described (Park et al, 2010). Mdm2 E3 ligase inhibitor (no. 373225) was purchased from Calbiochem.
Plasmids. Nore1A was excised from pACT2–Nore1A and inserted into EcoRI/SalI-digested pEntr3C to construct pEntr–Nore1A. The Nore1 expression plasmids were then generated using Gateway Technology (Invitrogen). Mouse Nore1A was expressed as a GST fusion protein in BL21 cells. Myc–HIPK1, GFP–HIPK1 and Flag–HIPK1 were described previously (Kim et al, 1998). The expression plasmid encoding H-Ras Q61L mutant was purchased from Upstate Biotechnology. Lentiviral pLKO.1 shRNA vectors against human HIPK1 were obtained from the DFCI-Broad RNAi Consortium.
In vitro pull-down assay. Myc-tagged HIPK1 constructs were translated in vitro using a TNT-coupled Reticulocyte Lysate System (Promega). Pull-down assays were performed by incubating equal amounts of GST or GST–Nore1A fusion proteins immobilized on glutathione-Sepharose beads with Myc–HIPK1. This mixture was placed onto a rocking platform for 2 h, washed three times with 20 mM Tris–HCl, pH 8.0, 150 mM NaCl and 0.5% Nonidet P40, and bound proteins were eluted and separated on an 8% SDS–polyacrylamide gel.
Anchorage-independent growth assay. A549 shHIPK1 cells were seeded into 0.3% agar containing 2 × DMEM and 10% FBS on top of a bed of 0.6% agar, in 35-mm dishes at a density of 5 × 103 cells per dish. Plating was done in duplicate. At 18–20 days after plating, colonies were photographed and counted in 10 randomly chosen fields. Colony numbers are presented as the means of the duplicate plates, and are representative of two independent experiments.
Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
Acknowledgments
This work was supported in part by a Korea Science and Engineering Foundation (KOSEF) grant (KRF-2008-313-C00605 and the Ubiquitome Research Program, 2011-00983 to C.Y.C.), and by a Mid-career Researcher Program through an National Research Foundation grant funded (no. 2009-0085548 to C.Y.C.). We also acknowledge support from the KOSEF grant funded by the Ministry of Education, Science and Technology (no. 2009-0063466 to S.-G.L. and S.-H.K.).
Author contributions: D.L., S.-J.P., J.K., Y.K. and C.Y.C. designed research. D.L., S.-J.P., K.S.S., J.P., S.B.L., S.-Y.P., H.J.L., J.-W.A., S.J.C. and S.-G.L. performed research. S.-H.K., D.-H.K. and J.K. analysed the data. D.L., Y.K. and C.Y.C. wrote the paper.
Footnotes
The authors declare that they have no conflict of interest.
References
- Aikawa Y, Nguyen LA, Isono K, Takakura N, Tagata Y, Schmitz ML, Koseki H, Kitabayashi I (2006) Roles of HIPK1 and HIPK2 in AML1- and p300-dependent transcription, hematopoiesis and blood vessel formation. EMBO J 25: 3955–3965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isono K, Nemoto K, Li Y, Takada Y, Suzuki R, Katsuki M, Nakagawara A, Koseki H (2006) Overlapping roles for homeodomain-interacting protein kinases hipk1 and hipk2 in the mediation of cell growth in response to morphogenetic and genotoxic signals. Mol Cell Biol 26: 2758–2771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khokhlatchev A, Rabizadeh S, Xavier R, Nedwidek M, Chen T, Zhang XF, Seed B, Avruch J (2002) Identification of a novel Ras-regulated proapoptotic pathway. Curr Biol 12: 253–265 [DOI] [PubMed] [Google Scholar]
- Kim YH, Choi CY, Lee SJ, Conti MA, Kim Y (1998) Homeodomain-interacting protein kinases, a novel family of co-repressors for homeodomain transcription factors. J Biol Chem 273: 25875–25879 [DOI] [PubMed] [Google Scholar]
- Kondo S et al. (2003) Characterization of cells and gene-targeted mice deficient for the p53-binding kinase homeodomain-interacting protein kinase 1 (HIPK1). Proc Natl Acad Sci USA 100: 5431–5436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Kang SI, Lee SY, Zhang XF, Kim MS, Beers LF, Lim DS, Avruch J, Kim HS, Lee SB (2010) Tumor suppressor Ras association domain family 5 (RASSF5/NORE1) mediates death receptor ligand-induced apoptosis. J Biol Chem 285: 35029–35038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter AM, Pfeifer GP, Dammann RH (2009) The RASSF proteins in cancer; from epigenetic silencing to functional characterization. Biochim Biophys Acta 1796: 114–128 [DOI] [PubMed] [Google Scholar]
- Song MS, Song SJ, Kim SY, Oh HJ, Lim DS (2008) The tumour suppressor RASSF1A promotes MDM2 self-ubiquitination by disrupting the MDM2--DAXX--HAUSP complex. EMBO J 27: 1863–1874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Weyden L, Adams DJ (2007) The Ras-association domain family (RASSF) members and their role in human tumourigenesis. Biochim Biophys Acta 1776: 58–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.