Nature Immunology (2011) advance online publication. doi:; DOI: 10.1038/ni.2194
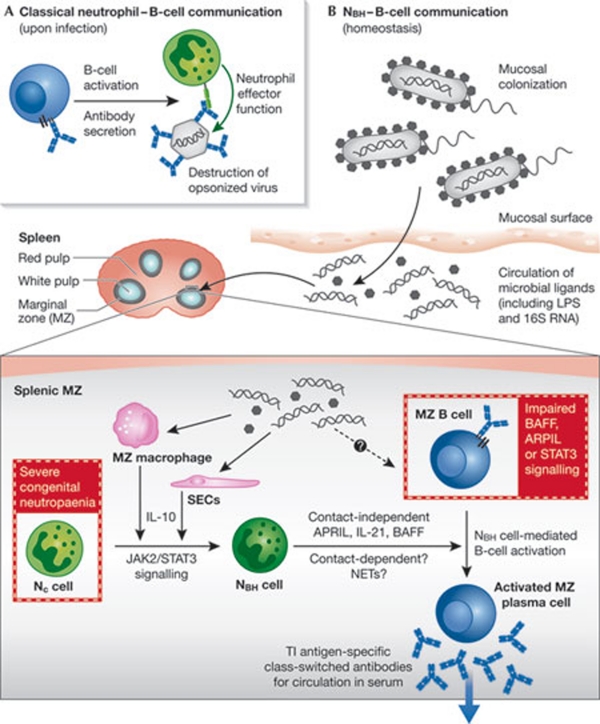
The effective elimination of pathogens requires cooperation between the innate and adaptive branches of the immune system. The innate branch mediates rapid inflammatory responses after infection, whereas highly specific adaptive responses emerge within a few days. The involvement of innate cells in mediating B-cell responses has been traditionally limited to the opsonization and destruction of antigen-coated pathogens (Fig 1A). However, both basophils (Chen et al, 2009) and eosinophils (Chu et al, 2011) have recently been shown to secrete B-cell stimulatory factors—such as BAFF, APRIL and IL-6—suggesting that innate cells can also influence B-cell activation. Similarly, although neutrophils are traditionally considered to be innate immune cells, they have been shown to influence adaptive responses during infection through the regulation of dendritic cell activation via alarmins (Yang et al, 2009) or IL-10 (Zhang et al, 2009). Moreover, in response to microbial products, murine neutrophils relocalize to the white pulp of the spleen, where they can encounter resident populations of lymphocytes (Kesteman et al, 2008). However, whether neutrophils regulate humoral immune responses was unknown. An impressive tour de force led by Andrea Cerutti and published this month in Nature Immunology, reveals that splenic neutrophils can function as professional helper cells for marginal zone B cells, leading to the generation of affinity-matured antibodies (Puga et al, 2011; Fig 1B).
Figure 1.
Cross-talk between neutrophils and B cells. (A) In response to infection, neutrophils (green) have been traditionally thought to opsonize pathogens that are coated with antibodies secreted by B cells (blue). (B) The newly identified B-helper neutrophil population (NBH, dark green) in the splenic marginal zone (MZ, grey) can activate MZ B cells (dark blue) to secrete antibodies against TI antigens. This probably occurs through the secretion of APRIL, BAFF and IL-21 in a contact-independent mechanism, although contact-dependent and/or neutrophil extracellular traps (NETs) might also play a role. Secreted antibodies are often class-switched and might enter the general circulation to provide basal innate immunity against microbial pathogens. NBH cells probably arise from circulatory neutrophils (Nc) as a result of JAK2 and STAT3 signalling, in response to IL-10 secretion by sinusoidal endothelial cells (SECs) and/or macrophages. This might be triggered by microbial ligands present in the general circulation that are translocated across mucosal surfaces after bacterial colonization. Patients with severe congenital neutropenia have reduced levels of antibodies against TI antigen, and patients with altered signalling in response to BAFF, APRIL and IL-21 have impaired MZ B-cell development (both highlighted in red boxes). LPS, lipopolysaccharide; TI, T-cell-independent.
The study begins by analysing the distribution of neutrophils in secondary lymphoid tissue sections from individuals without inflammation or infection. Under these conditions, although neutrophils are predominantly excluded from follicles, they are relatively abundant in regions proximal to the splenic marginal zone (MZ). The fact that such a distribution is conserved in both macaques and mice suggested that neutrophils in the peri-MZ might be functionally significant during homeostasis. Furthermore, this distribution is altered in pathological spleens, such that neutrophils infiltrate the follicular mantle and germinal centres.
Interestingly, the peri-MZ localization of neutrophils not only means that they are in an ideal location to respond to blood-borne antigens, but also renders them in close proximity to MZ B cells, which are classically associated with T-cell-independent antibody responses. In view of this, Puga and colleagues went on to show that this splenic neutrophil population—unlike those in general circulation (Nc)—are able to mediate the activation of IgM secretion from MZ B cells (Fig 1B). As a result, these cells were named B-helper neutrophils (NBH), and a detailed characterization of this population revealed the potential molecular mechanism underlying their capacity to mediate MZ B-cell activation. NBH have a higher expression of B-cell-stimulating molecules—such as BAFF, APRIL, IL-21 and CD40L—than do Nc cells. In line with this, NBH-cell-conditioned medium can activate MZ B cells, an effect that is abrogated when signalling through these receptors is blocked. However, as the extent of antibody secretion is greater after incubation with the NBH cells, contact-dependent mechanisms seem to also participate in MZ B-cell activation. Intriguingly, unlike Nc cells, the NBH population spontaneously forms DNA-containing neutrophil extracellular trap (NET)-like projections. Although similar structures have recently been associated with the ability to trigger Toll-like receptor 9 (TLR9)-mediated activation of dendritic cells and B cells in systemic lupus erythematosus (SLE; Lande et al, 2011), it is not clear whether NETs are involved in NBH-mediated MZ B-cell activation. In particular, it will be interesting to investigate the role of NETs as a potential source of immune complexes containing TLR9 ligands, which might facilitate B-cell activation (Leadbetter et al, 2002). Regardless, the identification of a population of neutrophils able to function as professional helper cells for MZ B cells uncovers an exciting new avenue for communication between the innate and adaptive immune networks.
But what is the consequence of NBH-mediated assistance on the MZ B-cell population? Follicular B-cell activation in response to T-cell-dependent antigen has been relatively well characterized and is often accompanied by the formation of germinal centres (MacLennan, 1994). Germinal centres have been traditionally associated with the diversification of the Ig genes through somatic hypermutation and subsequent selection of high-affinity clones, as well as the generation of immunological memory. However, although it has been reported that CD11clo dendritic cells promote the formation of plasmablasts from MZ B cells during systemic infection (Balázs et al, 2002), much less is understood about the impact of accessory cell help on the induction of T-cell-independent responses. Puga and colleagues showed that NBH cells trigger the expression of the Blimp 1 and XBP1 transcription factors and the surface marker CD38 in MZ B cells, which is indicative of plasmablast formation. Furthermore, in line with the upregulation of AID expression in MZ B cells in close proximity to NBH cells, the secreted antibodies were shown to have undergone class switch, favouring the generation of IgG2 and IgA. Importantly, in spite of normal levels of class-switched antibodies to T-cell-dependent antigens, patients with severe congenital neutropenia have decreased levels of IgA and IgG to microbial T-cell-independent antigens such as lipopolysaccharide. Interestingly, sequencing the antibodies secreted by NBH-activated MZ B cells also indicated that, at least in humans, they accumulate mutations as observed during somatic hypermutation. Thus, surprisingly, NBH cell assistance seems to trigger the diversification of antibodies from the MZ B-cell population, similarly to the influence of CD4+ T cells on follicular B cells.
The ability of NBH cells to mediate the secretion of class-switched antibodies from MZ B cells raises questions as to the origin of this population. When NC cells are exposed to IL-10, they upregulate the expression of mRNA encoding BAFF and APRIL, and become inducible NBH-like cells. The generation of this inducible population requires signalling through JAK2 and STAT3. NBH in the splenic MZ are in close proximity to sinusoidal endothelial cells, which secrete IL-10 and various neutrophil-attracting chemokines in response to microbial ligands. On this basis, Puga and colleagues postulate that microbial ligands—which might enter general circulation after systemic translocation across mucosal surfaces (Clarke et al, 2010)—trigger both reprogramming and chemotactic signals to Nc cells, resulting in the formation of NBH cells. In line with this concept, the splenic NBH population is established early in fetal life, but is greatly enhanced two days after birth, coincident with mucosal colonization by bacteria. Moreover, mice that are either germ-free or unable to mediate TLR signalling, have fewer NBH cells. In the light of these observations, NBH cells are suggested to stimulate the generation of class-switched antibodies to T-cell-independent antigens from MZ B cells in the steady state, providing individuals with an innate layer of antimicrobial antibody defence.
Several intriguing questions are raised by this study that will remain the challenge of future work. Such issues include the identification of the source of the initial signal that triggers the generation of the NBH cell population and uncovering the mechanism(s) by which NBH-mediated MZ B-cell activation is regulated. Nonetheless, this exciting study not only defines new communications between branches of the immune system, but also opens potential therapeutic avenues involving the manipulation of neutrophil populations to enhance basal immunity.
References
- Balázs M et al. (2002) Immunity 17: 341–352 [DOI] [PubMed] [Google Scholar]
- Chen K et al. (2009) Nat Immunol 10: 889–898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu VT et al. (2011) Nat Immunol 12: 151–159 [DOI] [PubMed] [Google Scholar]
- Clarke TB et al. (2010) Nat Med 16: 228–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesteman N et al. (2008) J Leukoc Biol 83: 640–647 [DOI] [PubMed] [Google Scholar]
- Lande R et al. (2011) Sci Transl Med 3: 73ra19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leadbetter EA et al. (2002) Nature 416: 603–607 [DOI] [PubMed] [Google Scholar]
- MacLennan IC (1994) Annu Rev Immunol 12: 117–139 [DOI] [PubMed] [Google Scholar]
- Puga et al. (2011) Nat Immunol [Epub ahead of print] doi:; DOI: 10.1038/ni.2194 [DOI] [Google Scholar]
- Yang D et al. (2009) Trends Immunol 30: 531–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X et al. (2009) Immunity 31: 761–771 [DOI] [PubMed] [Google Scholar]