Abstract
Introduction:
Dengue, an acute viral disease transmitted by Aedes mosquitoes, is highly endemic in many tropical and subtropical areas of the world. Neurological complications of dengue infection have been observed more frequently in the recent past and some studies highlighted varied neurological complications arising in the course of dengue illness. In this retrospective study, we report various neurological complications observed during the last 2 years in patients of dengue fever.
Materials and Methods:
The patients presenting with neurological complications with positive serology (IgM antibody) for dengue infection were consecutively recruited from the Department of Neurology/Medicine from a tertiary center of Lucknow, India. These patients were subjected to a detailed clinical evaluation, laboratory assessment including blood count, hematocrit, coagulation parameters, biochemical assays, serology for dengue fever, enzyme-linked immunosorbent assay for human immunodeficiency virus and other relevant investigations.
Results:
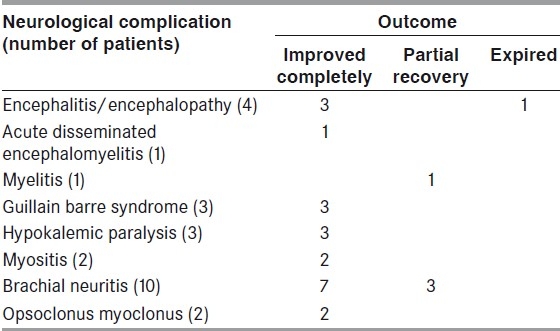
Twenty-six patients with neurological complications associated with confirmed dengue infection were observed during the last 2 years. Eighteen of these patients were male. Of the 26 patients, 10 patients were suffering from brachial neuritis, four patients had encephalopathy, three patients were consistent with the diagnosis of Guillain Barre syndrome, three patients had hypokalemic paralysis associated with dengue fever and two patients had acute viral myositis. Opsoclonus-myoclonus syndrome was diagnosed in two patients, myelitis in one patient and acute disseminated encephalo-myelitis also in one patient.
Conclusion:
Dengue fever was associated with widespread neurological complications. Brachial neuritis and opsoclonus-myoclonus syndrome were observed for the first time in this study.
Keywords: Brachial neuritis, dengue fever, hypokalemic paralysis, myositis, neurological complications
Introduction
Dengue fever is the mosquito-transmitted, arboviral infection mainly found in tropical and subtropical countries. Around 2.5 billion population worldwide is at risk of dengue infection, and its endemic zone comprises more than 100 countries of the world.[1] Dengue infection is caused by any of the four antigenically related distinct serotypes.[2] The clinical presentation of dengue infection has a wide spectra, ranging from mild clinical febrile illness to severe life-threatening situations like dengue hemorrhagic fever and dengue shock syndrome. In recent years, the virological characteristics of dengue viruses have been changing, resulting in widespread neurological complications. The neurological complication in dengue infection has been hypothesized through three pathogenic mechanisms: (1) concerned with neurotropism leading to encephalitis, meningitis, myositis and myelitis, (2) systemic complications resulting in encephalopathy, stroke and hypokalemic paralysis and (3) postinfectious immune-mediated acute disseminated encephalomyelitis, Guillain Barre syndrome and optic neuritis.[3] In this retrospective study from a tertiary center of Lucknow, India, we described various neurological complications associated with dengue fever observed in the last 2 years. We also compared our observations with the previous literature. We described in detail along with case illustrations of brachial neuritis and opsoclonus myoclonus syndrome as these were interesting and novel observations and summarized the other neurological manifestations.
Materials and Methods
In this retrospective study, conducted in a tertiary center of Lucknow, India, we analyzed the patients of dengue infection presenting with various neurological manifestations. All the patients of dengue fever presenting with neurological manifestations, admitted in the Department of Neurology/Department of Medicine from July 2008 to September 2010, were included in this analysis. This study was approved by the institutional ethical committee. All the patients of dengue fever presenting with neurological manifestations were included in the study. The dengue fever was diagnosed on the basis of the positive serum IgM antibody to dengue fever. The serum IgM antibody were analyzed by the enzyme-linked immunosorbent assay (ELISA) method using an IgM ELISA Kit by Panbio Invernis Medical Innovations, Australia Gmbh. It was a qualitative analysis and the titers were not measured. However, we performed serum IgM ELISA testing twice at 1-week apart in acute phase to reconfirm the dengue infection in all patients. The baseline characteristics, including age, sex, occupation and socioeconomic class, were noted. A detailed history, clinical evaluation and detailed neurological examination was performed in all patients. The muscle power was recorded and noted according to the Medical Research Council grading. Systemic complications of dengue fever including jaundice, lymphadenopathy, hepatoslenomegaly, cardiac, gastrointestinal, respiratory and hematological manifestations were specifically examined. The routine laboratory investigations including hemoglobin level, blood counts, platelets estimation, hematocrit, blood sugar, liver function test, renal function test, creatine kinase, prothrombin time, activated partial thromboplastin time and electrolytes were performed in each patient. Electrocardiogram and chest radiography were done in all patients. Nerve conduction studies, electromyography, electroencephalography, neuroimaging studies including magnetic resonance imaging of the cranium and spine were performed in selected patients as indicated by clinical presentation. Muscle biopsy was also performed in those patients considered to have myositis. The ELISA for dengue IgM was performed in serum in all patients. The cerebrospinal fluid analysis including IgM antibody for dengue fever, polymerase chain reaction study for Japanese encephalitis virus, herpes simplex virus and Mycobacterium tuberculosis were performed in the cerebrospinal fluid in patients suffering from encephalopathy/encephalitis. The ELISA for human immunodeficiency virus (HIV) was performed in all patients and viral studies for Ebstein Barre virus and varicella zoster were also performed in the serum of patients presenting with brachial neuritis. The antinuclear antibody, rheumatoid factor and antiphospholipid antibodies were also carried out to exclude autoimmune diseases. The outcome was defined at the end of 3 months into death, partial recovery when patient remains dependent for activities of daily living and complete recovery when became independent for activities of daily living.
Results
We observed 26 patients of dengue infection presenting with various neurological complications who were admitted in our institution in the last 2 years [Figure 1]. The age of patients ranged from 11 to 60 years (mean age, 29.08 years). Eighteen patients were male. The demographic and clinical profile of these patients is described in Table 1. In our study, we found varied neurological manifestations involving almost all parts of the nervous system. We categorized our observations into three groups on the basis of the possible pathogenic mechanism: (1) neurotropic complications – encephalitis, myelitis, myosistis, (2) systemic complications – hypokalemic paralysis and (3) postinfectious immune mediated – acute disseminated encephalomyelitis, Guillain Barre syndrome and opsoclonus myoclonus syndrome.
Figure 1.
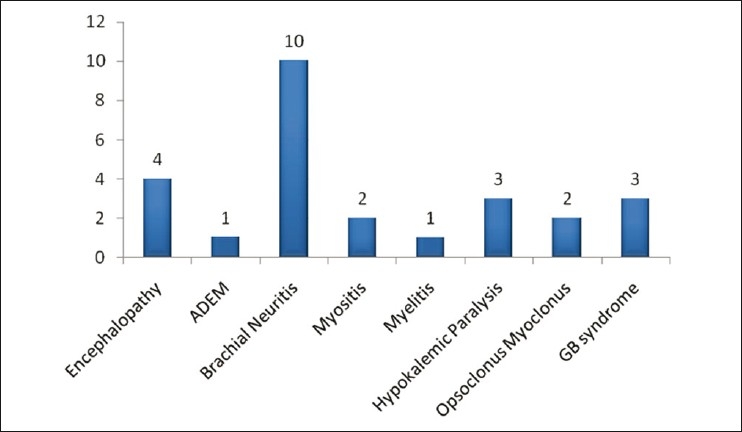
Frequency of different neurological complications in the study group
Table 1.
Demographic and clinical profile of the study patients
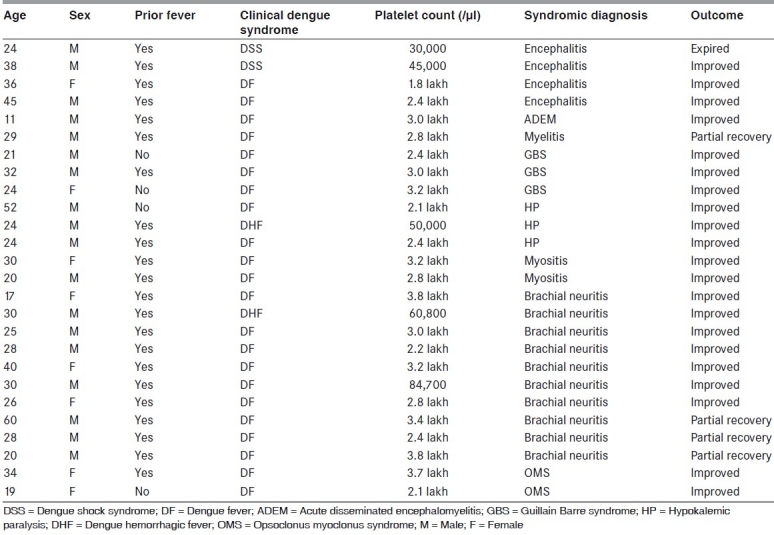
Regarding general characteristics of dengue infection, two patients were suffering from dengue shock syndrome, two patients from dengue hemorrhagic fever and the remaining patients had dengue fever. Petechial rash was seen in four patients (15.3%). Thrombocytopenia was noted in five patients (mean value, 41,600/mm3). The hepatosplenomegaly was found in two patients and renal failure manifested with high blood urea and serum creatinine in two patients.
Among neurotropic complications, we observed four patients of encephalitis, one of myelitis and two patients of myositis. In systemic complications, we managed three patients of hypokalemic paralysis and, in immune-mediated complications, 10 patients had brachial neuritis, one patient had Acute disseminated encephalomyelitis, three patients had Gulliain barre syndrome and two patients were diagnosed as having opsoclonus myoclonus syndrome.
Encephalopathy
Four patients (15.3%) presented with dengue encephalitis. All of them had fever, headache and severe myalgia for the initial 2–3 days followed by the development of seizures and altered sensorium. Two patients presented with epilepsia partialis continua along with intermittent focal with secondarily generalized seizures. Dengue shock syndrome with severe thrombocytopenia was observed in two patients. Cerebrospinal fluid analysis revealed normal findings in two patients and pleocytosis (30–40 cells, all lymphocytes) with normal values of proteins in the remaining two patients. The cerebrospinal fluid dengue IgM antibody was positive only in two patients, despite being positive in the serum in all four patients.
Myelopathy
One patient suffered from acute myelitis. He manifested with a 3-day history of acute quadriparesis with bladder and bowel involvement. Magnetic resonance imaging of the cervicothoracic spine demonstrated hyperintense signals on T2-weighted images extending from the lower cervical segments till the midthoracic spinal cord.
Myositis
Two patients suffered from acute myositis. Both patients presented with severe myalgia and progressive muscle weakness in both upper and lower limbs, mainly in symmetrical, proximal distribution. Both patients had raised temperature and their calves were tender on palpation. Serum creatine phoshpokinase was significantly elevated (mean value, 10,634 IU/L). Electromyography was suggestive of a myopathic pattern. Muscle biopsy revealed inflammatory cells on the histopathological study [Figure 2].
Figure 2.
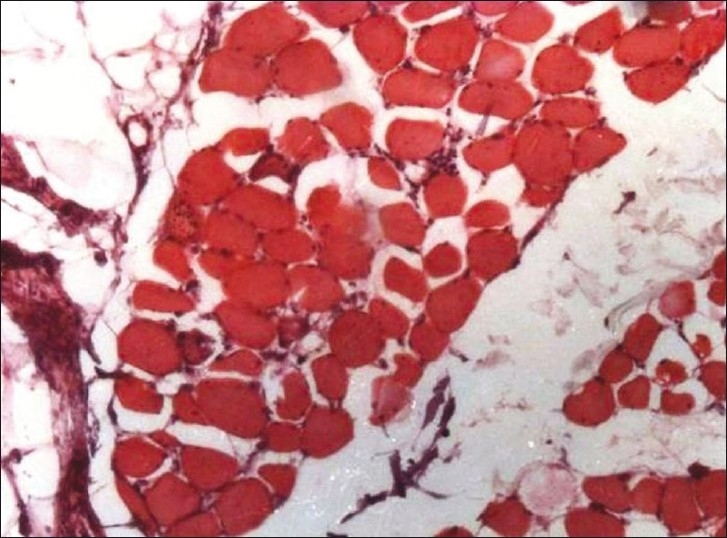
Muscle biopsy showing inflammatory cell infiltrate indicative of myositis
Peripheral neuropathy
Three patients (11.5%) patients were diagnosed as Guillain barre syndrome. Two of them had significant respiratory involvement and required prolonged ventilatory support. Neurophysiological studies revealed acute sensory motor axonal neuropathy (ASMAN) in two patients and demyelinating changes suggesting Acute inflammatory demyelinating polyradiculoneuropathy in the remaining one patient.
Acute disseminated encephalomyelitis
One patient presented with features suggestive of acute disseminated encephalomyelitis. He presented with high fever, severe generalized headache and vomiting from the last 4 days followed by progressive deterioration of the sensorium with two episodes of seizures on the day of admission to our hospital. On examination, his vitals were stable and he was stuporous. The spasticity was present in all four limbs with brisk deep tendon reflexes and upgoing plantars. Magnetic resonance imaging of the cranium and cervical spine depicted mutifocal predominant white matter lesions on T2-weighted images with involvement of the thalamus.
Brachial neuritis
There were 10 patients who presented with acute onset predominantly proximal weakness of the upper limb, preceded by a history of moderate to severe pain in the shoulder region. The diagnosis of brachial neuritis was established. The history of a febrile illness was present and dengue serology was positive in all patients. Age ranged from 27 to 60 years (mean age, 37.42 years). Of them, seven patients were male. The average duration between abatement of dengue infection and onset of clinical features of brachial neuritis in the form of neuropathic pain, weakness and atrophy of proximal musculature in the upper limbs was 14.85 days [Figure 3]. Six of 10 patients had right upper limb involvement. Neurophysiological evaluation, on stimulation of the proximal nerves of the upper limbs (axillary, musculocutaneous, long thoracic, suprascapular, radial, median, ulnar), revealed axonal changes in seven patients and demyelinating abnormality in the remaining three patients [Figure 4].
Figure 3.
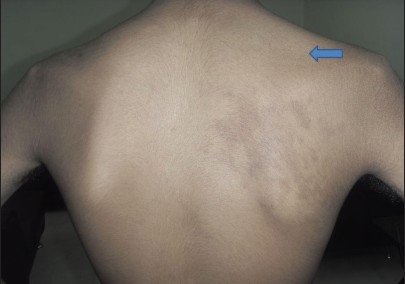
Arrow showing atrophy of supraspinatus and infraspinatus muscle on the right arm region in a patient of brachial neuritis
Figure 4.
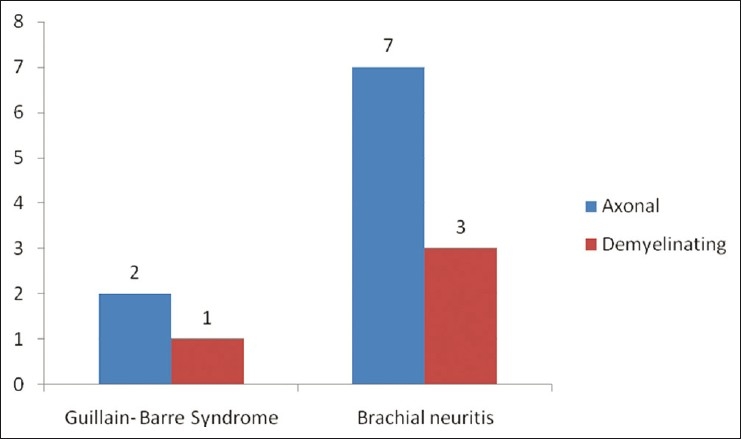
Clinical and neurophysiological finding in Guillain barre syndrome and brachial neuritis
Hypokalemic paralysis
Hypokalemic paralysis associated with dengue fever was seen in three patients. All patients were male. All of them presented with acute onset quadriparesis with 0–1/5 power (Medical Research Council grade) in a span of 2 days. All these patients had a severe degree of hypokalemia, serum potassium ranges from 1.0 to 2.1 meq/L (mean, 1.66). Electrocardiogram revealed features suggestive of hypokalemia as evidenced by the presence of U-wave [Figure 5].
Figure 5.
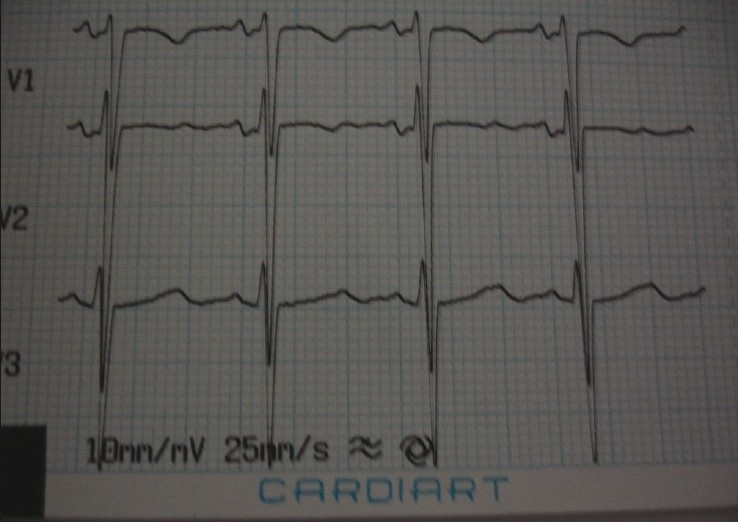
Electrocardiogram of the patient revealing U-waves suggestive of hypokalemia
Opsoclonus myoclonus syndrome
Opsoclonus myoclonus syndrome was confirmed in two patients associated with dengue fever. Both patients were female. The first patient developed acute onset tremulousness in the whole body along with involuntary chaotic rapid saccadic eye movements in the postpartum phase [Video]. At the same time, the patient had repetitive axial myoclonus.
The second patient presented with gait ataxia with chaotic, bilateral conjugate multivectoral eye movements suggestive of opsoclonus. Cerebrospinal fluid analysis and magnetic resonance imaging of the cranium did not reveal any abnormality.
Treatment and outcome
All patients received primarily a symptomatic treatment with special care for maintenance of fluid and electrolyte imbalance. Intravenous methylprednisolone and oral prednisolone were given to patients of myelitis, ADEM, brachial neuritis and myositis. Potassium supplementation in the standard regimen was given to patients of hypokalemic paralysis, IV Ig in standard dose of 0.4 gm/kg/day for 5 days for Guillain barre syndrome patients and clonazepam was prescribed to patients having opsoclonus myoclonus. Antiepileptics and other supportive treatment were given to patients having seizures and encephalopathy.
Of four encephalopathy/encephalitis patients, one could not be saved. All patients of ADEM, myositis, hypokalemic paralysis, Guillain barre syndrome and opsoclonus myoclonus had complete recovery. Residual disability was present in one patient having myelitis and in three patients of brachial neuritis [Table 2].
Table 2.
Outcome of patients having neurological complication in dengue fever
Case illustration: Opsoclonus myoclonus syndrome
A 24-year-old female, 2-months postpartum phase having full-term vaginal delivery presented with acute onset tremulousness of the whole body and involuntary chaotic eye movements that persisted even during sleep. The patient had difficulty in walking with intermittent arrhythmic muscle contractions, suggestive of myoclonic jerks. There was a preceding history of febrile illness, headache, vomiting, arthralgia and myalgia. General and other systemic examination was normal. Neurological examination revealed that the patient was conscious, in an oriented state and had normal speech. The ocular examination revealed chaotic, bilateral conjugate multivectoral eye movements that were present in all meridians, suggestive of opsoclonus. There was presence of axial myoclonic jerks, which increased on sitting or standing. The gait was ataxic in nature. Other parts of the neurological examination did not reveal any abnormality. History of vaccination was absent.
Investigations including hemogram, platelet count and peripheral blood smear examination were within normal limits. Biochemical evaluation revealed the following values: Serum bilirubin 1.7 mg/dl, serum glutamic pyruvate transaminase 25 Iu/L, serum alkaline phosphatase 127 Iu/L and serum protein 6.6 gm% with no evidence of reverse albumin globulin ratio. The renal function test did not reveal any abnormality. Thyroid examination was normal. Cerebrospinal fluid showed five cells, all lymphocytes, normal values of proteins with normal sugar. X-ray chest and whole abdomen ultrasound did not show any abnormality. Magnetic resonance imaging of the cranium was essentially normal. ELISA IgM antibody for dengue virus was positive in serum. Serological test for Epstein barre virus, varicella zoster, Japanese encephalitis, chikangunia, malaria and typhoid were negative. There was no evidence of hematopoetic malignancy. Paraneoplastic syndrome antibodies including anti-Yo, anti-Hu and anti-Ri were negative. After interpretation of complete clinical and laboratory parameters, opsoclonus myoclonus syndrome associated with dengue infection was considered. The patient was prescribed clonazepam in appropriate dosage and showed good clinical response.
Case illustration: Brachial neuritis
A 28-year-old, right-handed young man presented to our hospital with a 3-day history of moderate to severe pain in his right shoulder. The pain was burning in nature and radiating to the right arm. He also developed progressive weakness of the right upper limb in the form of difficulty in raising his hand above the shoulder. There was no history of any trauma, injury or vaccination. However, he had a history of moderate to high-grade fever with severe bodyache, retroorbital pain and arthralgia 10 days prior to development of the right shoulder pain. The fever subsided and he became asymptomatic over 2-3 days with some treatment. The examination was unremarkable except flaccid, proximal weakness of the right upper limb. After few days, he also developed wasting of muscles prominently involving the deltoid, supraspinatus and infraspinatus muscles on the right side.
He was considered to be a case of brachial neuritis and neurophysiological evaluation, including motor/sensory evaluation of the axillary nerve, musculocutaneous nerve, suprascapular nerve, long thoracic nerve, radial nerve, median and ulnar nerves, were performed, which revealed reduced amplitudes suggesting axonal degeneration. Later on, electromyographic evaluation was also carried out, which demonstrated spontaneous activity in the right deltoid, supraspinatus, biceps brachi, brachioradialis, abductor pollicis brevis and infraspinatus muscles and suggested denervation pattern. Magnetic resonance imaging of the cervical spine was normal. The ELISA for IgM antibodies to dengue virus in serum was positive. To reconfirm, we repeated IgM ELISA testing after 1 week, which was also positive. The viral studies (IgM antibody) for herpes virus, Japanese encephalitis virus, zoster virus and Ebstein barre virus were negative. The antinuclear antibody, rheumatoid factor and antiphosphilipid antibodies were also negative. The routine laboratory parameters, including renal functions, liver functions, hemogram, electrolyte and blood sugar, were in the normal range.
He was diagnosed as a case of brachial neuritis associated with dengue fever and prescribed prednisolone in the dosage of 1 mg/kg body weight along with amitriptyline. After 3 months of follow-up, the patient showed remarkable recovery and was independent for his daily activities.
Discussion
In the present study, varied neurological manifestations were observed in association with dengue fever. Demographically, patients belonged to all ages, and males outnumbered females by the factor of 1.7. Various studies mentioned age range 18–35 years (mean, 27 years), 5–65 years and male sex predominance in a large cohort related to neurological complications associated with dengue infection.[3,4] The true incidence of neurological complications associated with dengue fever are not clearly mentioned in the literature. The reported papers are mainly hospital-based studies and also neurological complications arising due to dengue fever is recently observed more frequently. In one study, 41 cases of neurological complications arising due to dengue fever have been reported. These patients had both central and peripheral neurological complications.[5] Misra et al. included 17 patients of neurological complications in his prospective hospital-based study, Wasay et al. reported six patients of neurological manifestations and Solomon et al. mentioned nine patients of encephalitis associated with dengue fever.[4,6,7]
In the last few years, numerous neurological complications related to dengue fever have been reported and neurological complications lead to significant morbidity and mortality. Neurological complications occur in 0.5–6% of the cases with dengue fever.[8] Both direct (neurotropism) and immunological mechanisms are responsible for neurological manifestations in dengue infection. Dengue antigen has been detected in the brain in some patients of dengue encephalitis.[9] Dengue encephalopathy could be caused by cerebral edema, cerebral hemorrhage, hyponatremia, hypoxia, renal and hepatic insult. Immune-mediated disorders including Guillain barre syndrome, acute disseminated encephalomyelitis and neuralgic amyotrophy, which is a new observation, are explained on the basis of autoimmunity, molecular mimicry or nonspecific activation of autoreactive T cell clones leading to destruction of the myelin sheath/self-antigens.[3,10]
In our study, we reported four patients of dengue encephalitis. Verma et al. recently reported a case of epilepsia partialis continua in a young woman suffering from dengue encephalitis. She presented with encephalitic syndrome of headache, vomiting, altered sensorium and intractable seizures.[11] In a study from Thai hospital, 18% children with encephalitis were found to have a positive dengue serology.[12] Misra et al. gave the description of 11 patients of encephalopathy with dengue infection.[6] Solomon et al. reported nine patients of dengue encephalitis. The diagnosis was based on positive antibody to dengue infection in sera and clinical presentation of focal neurological deficits, seizures and cerebrospinal fluid pleocytosis.[7]
We got two patients of opsoclonus myoclonus syndrome associated with dengue fever. Opsoclonus can be defined as saccadic stability disorder consisting of involuntary arrhythmic multidirectional high-amplitude conjugate saccades. Opsoclonus is often accompanied by diffuse or local myoclonus and truncal ataxia along with other cerebellar signs. Opsoclonus myoclonus syndrome is a rare neurological condition of unknown cause that appears to be the result of an autoimmune process involving the nervous system.[13] It is one of the few paraneoplastic syndromes that occur in both children and adults. In children, it is often associated with neuroblastoma and in adults, it is presumed to be of viral infection, e.g. Epstein barr, Coxsackie B or enterovirus, etc.[14] Till date, to the best of our knowledge, opsoclonus myoclonus syndrome has not been reported associated with dengue fever. In our patients, opsoclonus myoclonus syndrome could be explained on the basis of postinfectious immune-mediated disorder.
In the last few years, few case reports of acute disseminated encephalomyelitis in relation to dengue fever have been reported. The autopsy-proven case of acute disseminated encephalomyelitis has been reported in a case report. This patient had hemorrhagic demyelinating lesions and authors explained on the basis of thrombocytopenia.[15]
Acute myelitis as a consequence of dengue fever has been published through anecdotal case reports. Rajmundo et al. reported a case of acute myelitis where they were able to isolate the antigen from the central nervous system. This was a 58-year-old male who presented with fever, thrombocytopenia followed by acute paraperesis. This case report suggests that dengue virus has a propensity to directly invade the spinal cord, causing myelitis.[16] In another report, a case was mentioned of a 53-year-old male suffering from paraplegia due to epidural hematoma caused by thrombocytopenia associated with dengue fever.[17]
Guillain barre syndrome has been infrequently reported related to dengue fever. There were three patients of Guillain barre syndrome included in our study, acute sensory motor axonal neuropathy in two patients and acute inflammatory demyelinating polyneuropathy in one patient. Soares et al. discussed about seven cases of Guillain barre syndrome associated with dengue-positive IgM antibody in serum, with minimal clinical symptomatology. According to the authors, dengue infection should be routinely looked for in Guillain barre syndrome cases in an endemic zone.[18]
Various studies mentioned about involvement of the musculature leading to proximal weakness associated with dengue fever. Misra et al. in their study reported six patients of pure motor quadriparesis related to dengue fever. All these patients had normal findings on nerve conduction studies, myopathic pattern on electromyography with raised serum creatine phosphokinase suggestive of acute viral myositis. All patients showed improvement on therapy.[6] In a recently published study from a tertiary center of north India, seven patients of acute dengue viral myositis have been reported, in which authors highlighted that acute dengue viral myositis can present with fulminant variety requiring ventilatory support. These patients had different degrees of muscle weakness and were with or without respiratory involvement. They concluded that early respiratory involvement, severe myalgia and elevated serum creatine phosphokinase levels are indicators of severe acute dengue viral myositis.[19] We had two patients of dengue myositis whose clinical recovery was complete within 3 weeks.
In this study, we observed new neurological complications of dengue fever in the form of brachial neuritis. Brachial neuritis is a neurological disorder characterized by severe neuropathic pain, weakness and atrophy of the proximal musculature, mainly of the upper limbs, due to involvement of the brachial plexus. The various causative factors considered are viral infections, trauma, preceding strenuous exercise, immunization and autoimmune disorders.[20] Various viruses are implicated in causing brachial neuritis. A patient was reported of brachial neuritis associated with herpes zoster infection. The patient presented with vesicular rashes on the right arm and brachial plexopathy along with diminished power of extensors of the left hand.[21] Another patient of brachial neuritis was reported in association with hepatitis E virus.[22] There were 10 patients of brachial neuritis who presented with varying severity and different outcome in our study. We hypothesized that postdengue infection leads to autoimmune response against myelin sheath or other self-antigens causing brachial neuritis due to patchy demyelination of brachial plexus.
Hypokalemic paralysis has been recently reported in association with dengue fever. We also had three patients who developed acute pure motor quadriplegia with positive serology against dengue fever. All three patients had significant low serum potassium values and improved dramatically with potassium supplementation. Why hypokalemia occurs in dengue fever? Various putative mechanisms described in the literature are: (a) redistribution of potassium into the cells, (b) transient renal tubular abnormalities leading to increased urinary potassium wasting and (c) increased catecholamine levels secondary to infections, secondary insulin resistance leading to intracellular shift of potassium.[23]
The evaluation and investigations for dengue virus infection may involve detection of the virus, viral nucleic acid, antigens or antibodies (serology). In the initial 4–5 days, virus detection by culture, viral nucleic acid or antigen detection (NS 1) can be used to confirm dengue infection. However, after early acute phase, serology is preferred. Viral RNA nucleic acid detection by polymerase chain reaction assays are quite specific (100%) and sensitive (70%) in early acute phase, but they are more costly, more technical and not freely available everywhere. They may not be so useful in the later part of the illness when various neurological complications arise. As IgM antibody suggest recent infection, the detection of “dengue-specific” IgM antibody (serology) detection by MAC-ELISA (IgM antibody-capture enzyme-linked immunosorbent assay) is the preferred method for diagnosis after acute phase (after 5 days of fever onset).[24] Dengue fever can result in various neurological manifestations. Dengue infection should be considered and properly investigated in patients presenting with various neurological disorders without obvious etiology, especially if preceded by a febrile illness compatible with dengue fever.
Conclusions
Dengue fever is emerging as a disease of significant public health problem throughout the globe. Neurological complications of dengue infection are widespread and may involve almost all parts of the nervous system through various pathogenetic mechanisms. Along with various previously reported manifestations, in this study, we had described two new neurological complications of dengue fever: Opsoclonus myoclonus syndrome and brachial neuritis, which, to the best of our knowledge, have not been mentioned in the literature.
Videos available onlinewww.aian.org
Footnotes
Source of Support: Nil,
Conflict of Interest: Nil.
References
- 1.Dengue haemorrhagic fever; diagnosis, treatment, prevention, and control. Geneva: WHO; 1997. World Health Organisation. [Google Scholar]
- 2.Halstead SB. Dengue. Lancet. 2007;370:1644–52. doi: 10.1016/S0140-6736(07)61687-0. [DOI] [PubMed] [Google Scholar]
- 3.Murthy J. Neurological complications of dengue infection. Neurol India. 2010;58:581–4. doi: 10.4103/0028-3886.68654. [DOI] [PubMed] [Google Scholar]
- 4.Wasay M, Channa R, Jumani M, Shabbir G, Azeemuddin M, Zafar A. Encephalitis and myelitis associated with dengue viral infection clinical and neuroimaging features. Clin Neurol Neurosurg. 2008;110:635–40. doi: 10.1016/j.clineuro.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 5.Ferreira ML, Cavalcanti CG, Coelho CA, Mesquita SD. [Neurological manifestations of dengue: Study of 41 cases] Arq Neuropsiquiatr. 2005;63:488–93. doi: 10.1590/s0004-282x2005000300023. [DOI] [PubMed] [Google Scholar]
- 6.Misra UK, Kalita J, Syam UK, Dhole TN. Neurological manifestations of dengue virus infection. J Neurol Sci. 2006;244:117–22. doi: 10.1016/j.jns.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 7.Solomon T, Dung NM, Vaughn DW, Kneen R, Thao LT, Raengsakulrach B, et al. Neurological manifestations of dengue infection. Lancet. 2000;25(355):1053–9. doi: 10.1016/S0140-6736(00)02036-5. [DOI] [PubMed] [Google Scholar]
- 8.Hendarto SK, Hadinegoro SR. Dengue encephalopathy. Acta Paediatr Jpn. 1992;34:350–7. doi: 10.1111/j.1442-200x.1992.tb00971.x. [DOI] [PubMed] [Google Scholar]
- 9.Lum LC, Lam SK, Choy YS, George R, Harun F. Dengue encephalitis: A true entity? Am J Trop Med Hyg. 1996;54:256–9. doi: 10.4269/ajtmh.1996.54.256. [DOI] [PubMed] [Google Scholar]
- 10.Varatharaj A. Encephalitis in the clinical spectrum of dengue infection. Neurol India. 2010;58:585–91. doi: 10.4103/0028-3886.68655. [DOI] [PubMed] [Google Scholar]
- 11.Verma R, Varatharaj A. Epilepsia partialis continua as a manifestation of dengue encephalitis. Epilepsy Behav. 2011;20:395–7. doi: 10.1016/j.yebeh.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 12.Wiwanitkit V. Magnitude and pattern of neurological pathology in fatal dengue hemorrhagic fever: A summary of Thai cases. Neuropathology. 2005;25:398. doi: 10.1111/j.1440-1789.2005.00653.x. [DOI] [PubMed] [Google Scholar]
- 13.Garg RK, Kar AM, Dixit V. Opsoclonus myoclonus syndrome in an adult: A case report and response to clonazepam. Indian J Opthamol. 1996;44:101–2. [PubMed] [Google Scholar]
- 14.Blaes F, Pike MG, Lang B. Autoantibodies in childhood Opsoclonus myoclonus syndrome. (221-6).J Neuroimmunol. 2008:201–202. doi: 10.1016/j.jneuroim.2008.05.033. [DOI] [PubMed] [Google Scholar]
- 15.Sundaram C, Uppin SG, Dakshinamurthy KV, Borghain R. Acute disseminating encephalomyelitis following dengue haemorrhagic fever. Neurol India. 2010;58:599–601. doi: 10.4103/0028-3886.68666. [DOI] [PubMed] [Google Scholar]
- 16.Leão RN, Oikawa T, Rosa ES, Yamaki JT, Rodrigues SG, Vasconcelos HB, et al. Isolation of dengue 2 virus from a patient with central nervous system involvement (transverse myelitis) Rev Soc Bras Med Trop. 2002;35:401–4. doi: 10.1590/s0037-86822002000400018. [DOI] [PubMed] [Google Scholar]
- 17.Singh P, Joseph B. Paraplegia in a patient with dengue. Neurol India. 2010;58:962–3. doi: 10.4103/0028-3886.73770. [DOI] [PubMed] [Google Scholar]
- 18.Soares CN, Cabral-Castro MJ, Peralta JM, Freitas MR, Puccioni-Sohler M. Oligosymptomatic dengue infection: A potential cause of Guillain Barre syndrome. Arq Neuropsiquiatr. 2008;66:234–7. doi: 10.1590/s0004-282x2008000200018. [DOI] [PubMed] [Google Scholar]
- 19.Paliwal VK, Garg RK, Juyal R, Husain N, Verma R, Sharma PK, et al. Acute dengue virus myositis: A report of seven patients of varying clinical severity including two cases with severe fulminant myositis. J Neurol Sci. 2011;300:14–8. doi: 10.1016/j.jns.2010.10.022. [DOI] [PubMed] [Google Scholar]
- 20.Mccarty EC, Tsairis P, Warren RF. Brachial neuritis. Clin Orthop. 1999;368:37–43. [PubMed] [Google Scholar]
- 21.Jeevarethinam A, Ihuoma A, Ahmad N. Herpes zoster brachial plexopathy with predominant radial nerve palsy. Clin Med. 2009;9:500–1. doi: 10.7861/clinmedicine.9-5-500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fong F, Illahi M. Neuralgic amyotrophy associated with hepatitis E virus. Clin Neurol Neurosurg. 2009;111:193–5. doi: 10.1016/j.clineuro.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 23.Jha S, Ansari MK. Dengue infection causing acute hypokalemic quadriparesis. Neurol India. 2010;58:592–4. doi: 10.4103/0028-3886.68657. [DOI] [PubMed] [Google Scholar]
- 24.Dengue guidelines for diagnosis, treatment, prevention and control new edition. Geneva: World Health Organization; 2009. WHO. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


