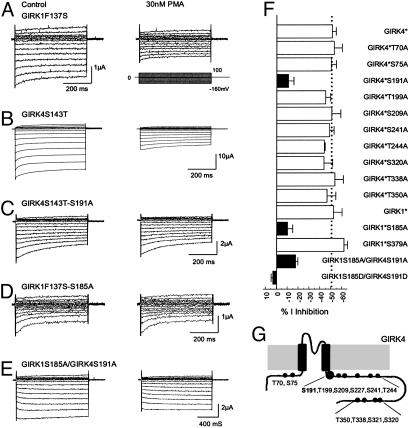
Fig. 3.
PMA sensitivity of homomeric and heteromeric GIRK1 and GIRK4. (A) Currents were recorded from an oocyte in the same condition as Fig. 1A. The GIRK1F137S currents were markedly inhibited by 30 nM PMA. (B) Similar current inhibition was seen in another oocyte injected with the GIRK4S143T cDNA. (C) When Ser-191 was substituted with an alanine in GIRK4S143T, the GIRK4S143T-S191A currents were barely inhibited by 30 nM PMA. Similar results were found in the GIRK1F137S-S185A mutant (D) and heteromeric GIRK1S185A/GIRK4S191A channel (E). (F) Identification of PKC phosphorylation sites by alanine-scanning mutagenesis. Ser-185 in the GIRK4 and Ser-191 in the GIRK4 were shown to affect the PMA sensitivity. Mutation of any one greatly eliminated the PMA sensitivity of the homomeric channels. Joint mutations of both also significantly diminished the PMA sensitivity of heteromeric GIRK1/GIRK4 channel. Mutations of other putative PKC phosphorylation sites had no effect (P < 0.01 for all filled bars). GIRK1*, GIRK1F137S; GIRK4*, GIRK4S143T. (G) Schematic of the GRIK4 subunit and locations of all serine/threonine residues studied.