Figure 4.
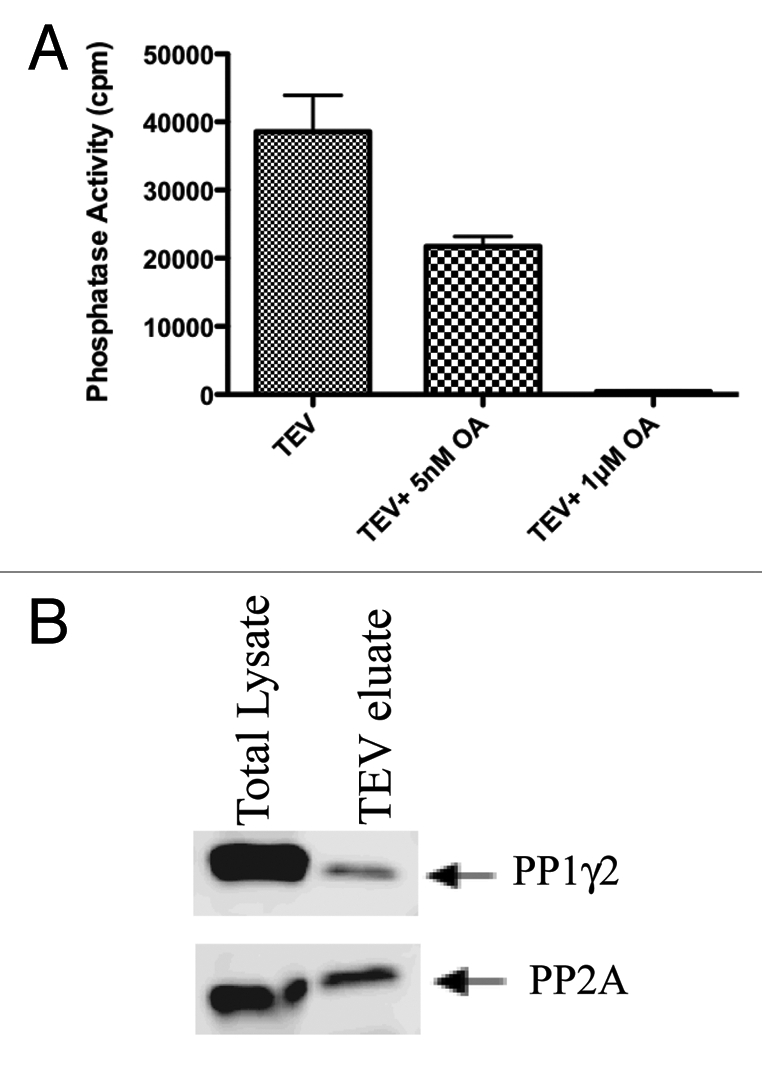
PP1 and PP2A bound to 14-3-3 are catalytically active. The TEV eluate isolated after first step of TAP purification was checked for the phosphatase activity of PP 1 and PP 2A as described in materials and methods. For protein phosphatase activity measurements, Phosphorylase a, a common substrate for both PP1 and PP2A was used. PP2A activity could be inhibited with 5 nM okadaic acid. The activity of both PP2A and PP1 could be inhibited to basal levels with 1 µM okadaic acid. (B) Protein gel blot analysis of the TEV eluate showed that both PP1 and PP2A interact with 14-3-3 in testis.
