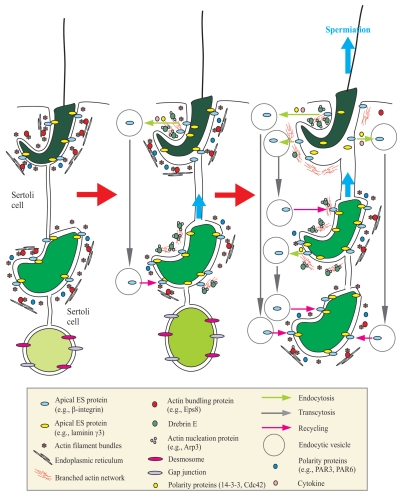
Figure 1.
A schematic drawing that illustrates an emerging concept regarding the role of actin-binding protein drebrin E in regulating spermiogenesis in the rat testis via its effects to recruit the actin nucleation protein Arp3 to the apical ES to facilitate junction restructuring during spermatogenesis. The left part in this figure illustrates intact apical ES (maintained by adhesion protein complexes such as integrin-laminin at the Sertoli cell-step 8–19 spermatid interface), gap junction and desmosome [at the Sertoli cell-step 1–7 spermatid interface] that confers proper adhesion of developing spermatids to the Sertoli cell in the seminiferous epithelium. Apical ES adhesion is conferred and strengthened by actin filament bundles sandwiched in between the cisternae of endoplasmic reticulum and the Sertoli cell plasma membrane, and this likely involves the presence of polarity proteins, such as PAR3 (partitioning-defective protein 3), PAR6.72 Highly organized F-actin filament bundles uniquely found at the apical ES are maintained by actin-bundling proteins, such as Eps8. During spermiogenesis, the transit of developing spermatids is facilitated by a surge in the expression of drebrin E, which recruits actin nucleation proteins (e.g., Arp3 in the Arp2/3 protein complex) to the apical ES to convert actin filament bundles into a branched network, causing the loss of “rigidity” of, but conferring “plasticity” to, the apical ES (see middle part). This thus destabilizes the apical ES, facilitating protein endocytosis, which is regulated by cytokines (e.g., TGFβ3 and TNFα) 8 and assisted by polarity proteins (e.g., 14-3-3, Cdc42).73,74 As spermiogenesis progresses, the elevated expression of drebrin E recruits more Arp3 to the apical ES, surrounding the head of elongated spermatids to further destabilize adhesion at the apical ES to facilitate the release of sperm at spermiation (i.e., degeneration of the apical ES at stage VIII of the epithelial cycle), and internalized apical ES proteins can be transcytosed and recycled to assemble “new” apical ES to anchor newly differentiated step 8 spermatids onto the epithelium (see right part). This thus provides an efficient physiological system to “re-use” many of the component proteins from the “old” apical ES site surrounding the head of step 19 spermatids to assemble the “new” apical ES in step 8 spermatids that arises during spermiogenesis. This emerging new concept is the basis for many functional studies in the future.