Figure 4.
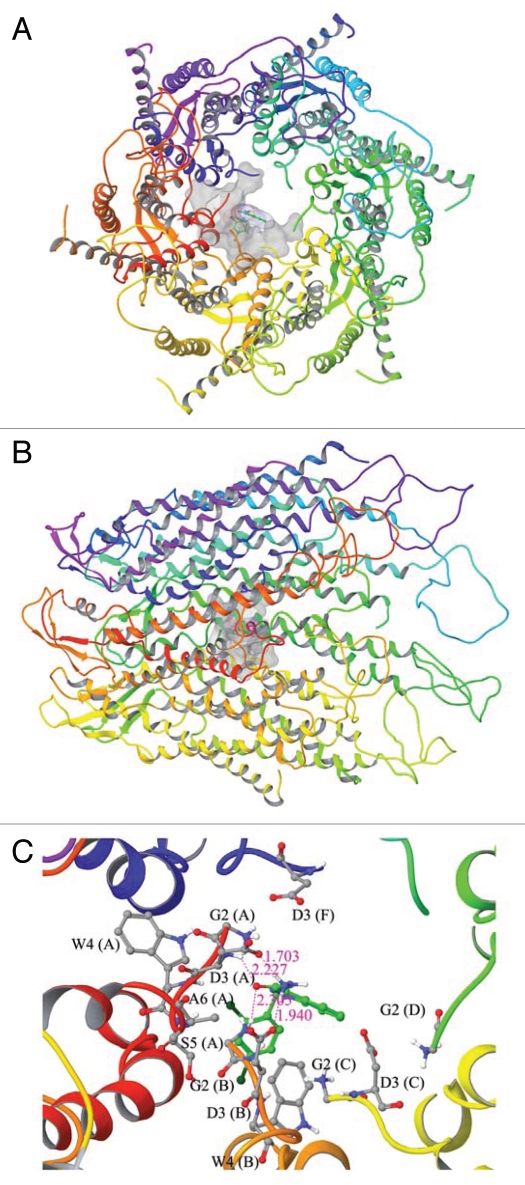
A molecular modeling study to assess the docked complex of adjudin with Cx43-based gap junction communication channel. (A) Topographic view of the Cx43-gap junction protein complex with adjudin. (B) Side view of the Cx43-gap junction protein complex with adjudin. (C) Ball and stick model for the interaction residues and secondary structure representation for protein between adjudin and Cx43. Docking of adjudin into Cx43 shows strong interactions in the pore lining residues of N-terminal helix (NTH). The amino acid residues such as Gly2 (G2, Chain B) and Asp3 (D3, Chain A and B) were involved in multiple hydrogen bond formation with adjudin (C). The backbone nitrogen atom of Gly2 (G2, Chain B) and side chain nitrogen atom of Asp3 (D3, Chain B) were shown to have hydrogen bond interaction with adjudin in the bond distances of 2.305 Å and 1.940 Å, respectively. Whereas the side chain oxygen of Asp3 (D3, Chain A) and backbone nitrogen atom of Asp3 (D3, Chain A) were also involved in hydrogen bond formation in the bond distance of 1.703 Å and 2.227 Å, respectively. In addition to hydrogen bonds, Gly2 (G2, Chain A, C, D and F), Asp3 (D3, Chain C), Trp3 (W3, Chain A and B), Ser5 (S5, Chain A) and Ala6 (A6, Chain A) were involved in non-bonded interaction to further stabilize the interaction. The docking score of this binding complex was −4.47 kcal/mol. The size of surface area for binding site and ligand were 978.172 Å and 290.038 Å, respectively. This docking result has shown that adjudin binds with pore lining N-terminal helix residues of chain (A–D) and (F) except chain (E). Hence, it has the possibility that adjudin can block the gating of this channel protein. From these docking studies, we have observed that large size of both binding site surface in BPA and ligand surface in adjudin makes very strong binding into N-terminal helix of Cx43. On the other hand, docking of estradiol-17β reveals very little docking energy when compared to docking of other two compounds, which indicates estradiol-17β can make very stable binding into Cx43-based GJ communication channel. It can also be noted that both NTH and TMH1 amino residues are involved in the binding pocket for estradiol-17β interactions. Our docking results disclose that each of the compounds is forming at least one hydrogen bond with the binding site residues in the Cx43-based communication channel. As a whole we concluded that all three compounds can regulate the function of Cx43 by binding with pore lining residues of this protein.