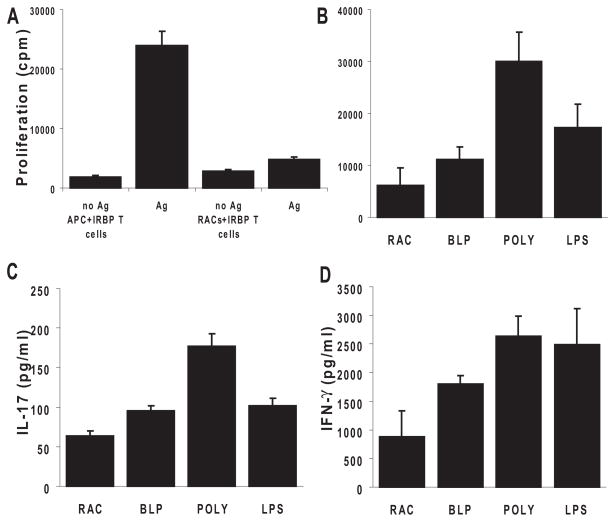
Figure 3.
RACs activated by TLRs shaped antigen-specific uveitogenic T-cell proliferation and differentiation into Th1 and Th17 cells. (A) Nonactivated RACs lack the ability to present antigen to IRBP-specific T cells compared with peripheral APCs. T cells (3 × 105) from in vivo IRBP1–20 –primed B6 mice were cultured with 1 × 105 syngeneic irradiated spleen cells or MMC-treated RACs in the presence of 10 μg/mL IRBP1–20; proliferation was measured by the incorporation of [3H]-thymidine (0.5–1 μCi/well) during the last 8 hours of the 72-hour incubation period. (B) PolyI:C-treated RACs were effective in presenting uveitogenic peptide, leading to the proliferation of IRBP1–20 –specific T cells. Cultured RACs were treated for 24 hours with TLR ligands (the same concentrations as in Fig. 1), washed, and MMC-treated before coculture with T cells. (C) TLR ligand-pre-treated RACs induced IRBP-specific T cells to produce proinflammatory cytokines. The experimental paradigm was as in (D). After 48 hours, the supernatants from triplicate cultures were pooled, and cytokines were measured by ELISA. Values are the mean ± SEM for three individual experiments.