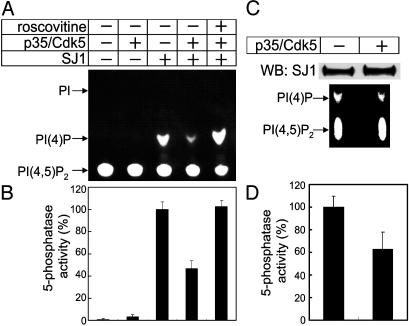
Fig. 3.
Phosphorylation of synaptojanin 1 by Cdk5 inhibits its 5-phosphatase activity. (A) Synaptojanin 1 was incubated with the p35/Cdk5 complex in phosphorylation conditions (see Fig. 2) in the presence or absence of the Cdk5 inhibitor roscovitine (20 μM). Phosphorylation mixtures and their controls were then added to assay tubes containing water-soluble PI(4,5)P2 substrates [diC8–PI(4,5)P2 and NBD6-PI(4,5)P2] and further incubated for 10–15 min at 37°C. PI(4,5)P2 cleavage was monitored by analysis of NBD6 fluorescence after TLC chromatography. Note that only PI(4)P is generated. In separate experiments (data not shown), the 5-phosphatase activity of synaptojanin 1 was monitored by the release of free phosphate from diC8-PI(4,5)P2 using a malachite green-based assay (47). (B) Pooled results of 5-phosphatase activity assays involving the TLC and the malachite green-based assay. (C) Antisynaptojanin 1 immunoprecipitates from rat brain extracts were incubated in the presence or absence of the p35/Cdk5 complex in phosphorylation conditions, and the resulting mixtures were analyzed for 5-phosphatase activity as described for A. The Western blot (Upper) indicates that the same amount of synaptojanin 1 was present in the two samples. (D) A result of 5-phosphatase activity measured by malachite green-based assay.