Abstract
Xylan, a hemicellulosic component of the plant cell wall, is one of the most abundant polysaccharides in nature. In contrast to dicots, xylan in grasses is extensively modified by α-(1,2)– and α-(1,3)–linked arabinofuranose. Despite the importance of grass arabinoxylan in human and animal nutrition and for bioenergy, the enzymes adding the arabinosyl substitutions are unknown. Here we demonstrate that knocking-down glycosyltransferase (GT) 61 expression in wheat endosperm strongly decreases α-(1,3)–linked arabinosyl substitution of xylan. Moreover, heterologous expression of wheat and rice GT61s in Arabidopsis leads to arabinosylation of the xylan, and therefore provides gain-of-function evidence for α-(1,3)-arabinosyltransferase activity. Thus, GT61 proteins play a key role in arabinoxylan biosynthesis and therefore in the evolutionary divergence of grass cell walls.
Keywords: type II cell walls, second-generation biofuels, dietary fiber
Cell walls provide shape and strength to different plant cell types and, moreover, constitute the majority of plant biomass. The cell wall composition of grasses, including the three most productive food crops, rice, wheat, and maize, and the energy crops miscanthus and sugarcane, diverged during evolution from dicots. A major distinguishing feature of grass cell walls is the prevalence and structure of the hemicellulosic component xylan (1). Xylan consists of a linear β-(1,4)-d-xylopyranose (Xylp) chain. It is most commonly substituted by arabinofuranose (Araf) on the C2- or C3-position in arabinoxylan (AX), and (4-O-methyl-) glucuronosyl side chains on the C2-position in glucuronoarabinoxylan (GAX) and glucuronoxylan (GX). The primary and secondary cell walls of grasses contain substantial amounts of GAX, which is also found in primary cell walls of dicots, but at much lower abundance (1, 2). In contrast, xylan in secondary cell walls of dicots is relatively abundant but devoid of arabinosyl side chains (2). The functional significance of the different side chains in planta is largely unknown. In grasses α-(1-3)–linked arabinofuranosyl substitutions can be esterified with p-coumaric or ferulic acid, the latter forming cross-links with other (G)AX chains (3) or with lignin (4). Cross-linking of cell-wall polymers is critical in limiting the digestibility of polysaccharides for bioenergy production and animal feed. In addition, AX has a role as dietary fiber in human foods, particularly in wheat flour, where it constitutes 65–70% of the nonstarch polysaccharide (5). The degree of arabinosylation and feruloylation of AX also determines whether it occurs as soluble or insoluble dietary fiber, which confer different benefits to human health (6).
In Arabidopsis thaliana (Arabidopsis), several glycosyltransferases of the GT43 and GT47 families have been shown to be involved in the biosynthesis of the xylan backbone, including IRX9, IRX10, and IRX14 (2). The only enzymes characterized so far that decorate the xylan backbone are members of the GT8 family, GUX1 and GUX2, which are required for glucuronosyl substitution in Arabidopsis stem (7).
We previously used a bioinformatic approach to identify candidate genes involved in AX biosynthesis, reasoning that the greater abundance and different structure of xylan in grasses compared with dicots should be reflected in the expression of xylan biosynthesis genes. Orthologs of the xylan backbone synthesis machinery were identified, but GT61 genes were shown to have the greatest grass expression bias of all of the GTs (8). However, experimental evidence for the involvement of GT61s in xylan biosynthesis is lacking. Here we demonstrate that rice and wheat GT61 family members are responsible for α-(1,3)-arabinosyl substitution of xylan, and thus name these proteins xylan arabinosyltransferases (XATs).
Results
Phylogenetic analysis of the GT61 enzymes showed that the family can be divided into three clades (Fig. S1A), of which only the divergent Clade C has been functionally characterized as Golgi-localized β-(1,2)-xylosyltransferases involved in N-glycosylation (9). Clade A is specific for higher plants with no representatives in Physcomitrella patens and Selaginella moellendorffii, and grass genes have undergone extensive duplication. Two Clade A GT61 genes, TaXAT1 and TaXAT2 (Fig. S1A), are abundantly expressed in wheat grain (10), which is consistent with their involvement in AX biosynthesis.
Xylan is synthesized in the Golgi apparatus and XAT activity has been found in wheat Golgi membranes (11, 12). To determine whether our wheat XAT candidates are Golgi proteins, we expressed a GFP fusion of TaXAT2 (TaXAT2-GFP) in tobacco-leaf cells. TaXAT2-GFP fluorescence was detected in punctate structures that colocalize with the Golgi-marker GONST1 (13) (Fig. S1B), locating XAT to the site of AX biosynthesis.
We have recently shown that six GT61 genes are expressed in wheat starchy endosperm and that TaXAT1 is much more highly expressed than any of the others, including the second most highly expressed, TaXAT2 (14). To investigate a role for GT61 genes in AX biosynthesis, RNAi constructs were therefore designed to suppress specifically TaXAT1 expression in the starchy endosperm of wheat. Although TaXAT2 is more expressed than TaXAT1 in whole-grain (10), in the starchy endosperm TaXAT2 expression is only about 12% of that of TaXAT1 (14) (Fig. S2A). The TaXAT1 RNAi construct resulted in suppression of the endogenous TaXAT1 transcript by 6- to 30-fold, with no consistent effect on TaXAT2 (Fig. S2 B and C).
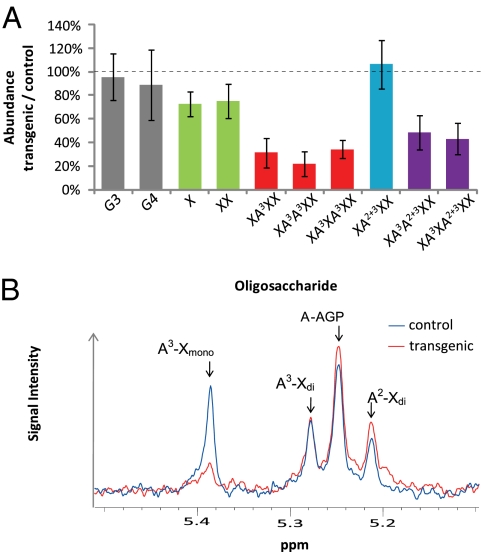
Endosperm cell-wall fractions from RNAi lines were analyzed by digestion with a GH11 endo-xylanase followed by quantification of the resulting xylan oligosaccharides by high-performance anion-exchange chromatography (HPAEC). The ratios of oligosaccharide abundance in TaXAT1 RNAi transgenic samples compared with their azygous control are shown averaged across independent lines (Fig. 1A; see Fig. S3 for structures of all substituted xylan oligosaccharides). Interestingly, the abundances of all oligosaccharides containing monosubstituted α-(1,3)–linked Araf (XA3XX, XA3A3XX, XA3XA3XX, XA3A2+3XX, XA3XA2+3XX) were substantially decreased (Fig. 1A), and these decreases were also highly significant within all lines (Table S1). None of the other oligosaccharides were consistently affected, although a smaller effect on unsubstituted oligosaccharides was observed in four lines (Fig. 1A and Table S1). A decrease in total AX was confirmed by monosaccharide analysis in two of these lines (Table S2). Cell-wall fractions isolated from endosperm samples were further analyzed by 1H-NMR; this confirmed the results from HPAEC showing a specific decrease in the peak corresponding to H1 of Araf α-(1,3)–linked to monosubstituted Xylp in AX, whereas the peaks corresponding to H1 of Araf α-(1,2)– or α-(1,3)–linked to di-substituted Xylp were unchanged (Fig. 1B). The structural changes to AX induced by the transgene resulted in the water-extractable percentage of total AX being decreased from 42 to 18% in line 2, and from 33% to 21% in line 3 (Table S2).
Fig. 1.
Analysis of xylan structure in endosperm samples from homozygous TaXAT1 RNAi transgenic wheat. (A) Oligosaccharide abundance (HPAEC peak area) from transgenic samples relative to corresponding azygous controls after xylanase digest; mean of five independent lines ± 95% confidence intervals. Columns for AX oligosaccharides are colored according to substitution with Araf: unsubstituted (green), monosubstituted only (red), di-substituted only (blue), and mono- and di-substituted (purple). Abundances of (1,3);(1,4)-β-glucan oligosaccharides [Glucose3 (G3) and Glucose4 (G4)] as result of simultaneous lichenase digest show no overall change. (B) 1H-NMR spectra for transgenic (red) and azygous control (blue) samples showing H1 signals for Araf in AX: α-(1,3)–linked to monosubstituted Xylp (A3-Xmono), α-(1,3)–linked (A3-Xdi), and α-(1,2)–linked (A2-Xdi) to di-substituted Xylp and for Araf in arabinogalactan peptide (A-AGP).
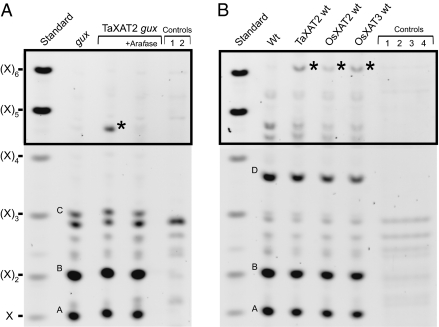
These results in transgenic wheat strongly suggest that GT61 family members of Clade A are responsible for arabinosylation of xylan. To analyze the biochemical activity of GT61s in an in vivo system without intrinsic arabinosyltransferase activity, we chose heterologous expression of epitope-tagged wheat TaXATs in Arabidopsis stem (Fig. S4A). To mimic the situation in wheat endosperm more closely, we transformed the gux1 gux2 double-mutant (gux), which lacks glucuronosyl substitutions in stem xylan (7). Stem-derived alcohol-insoluble residue (AIR) was digested with GH11 endo-xylanase and the resulting fragments analyzed using polysaccharide analysis by carbohydrate gel-electrophoresis (PACE). In addition to the characteristic oligosaccharides generated by the xylanase digest of gux xylan (X, XX, XXX) (7), an additional minor fragment clearly detectable between marker (X)4 and (X)5 was released from the TaXAT2 AIR (Fig. 2A). Further digestion of the xylan oligosaccharides with arabinofuranosidase GH62, which specifically removes C2- or C3-monosubstituted α-linked arabinofuranosyl substitutions (15), showed that the transgene-dependent oligosaccharide is arabinofuranosidase-sensitive (Fig. 2A). Given the migration in the gel and the specificities of the enzymes, we predicted the structure to have a degree of polymerization (DP) of 5, most probably being either XA3XX or XA2XX.
Fig. 2.
PACE analysis of xylan structure of XAT transgenic material after digestion with xylanase. (A) Xylan fingerprint of gux in comparison with transgenics expressing TaXAT2 in gux background; +Arafase: additionally digested with arabinofuranosidase. Controls: Undigested material of gux without (1) and with (2) transgene. (B) Xylan fingerprint of wild-type (Wt) in comparison with transgenics expressing TaXAT2, OsXAT2 or OsXAT3 in wild-type background. Controls: Undigested material of wt (1), TaXAT2 wt (2), OsXAT2 wt (3), and OsXAT3 wt (4). Standard: Xylosyl oligosaccharides (X)1–6. Boxed area shows five-times longer exposure of identical gel. Asterisks mark the oligosaccharide specific for the transgenic lines. Oligosaccharide assignment is as follows. A, X; B, XX; C, XXX; D, XU(4m)2XX.
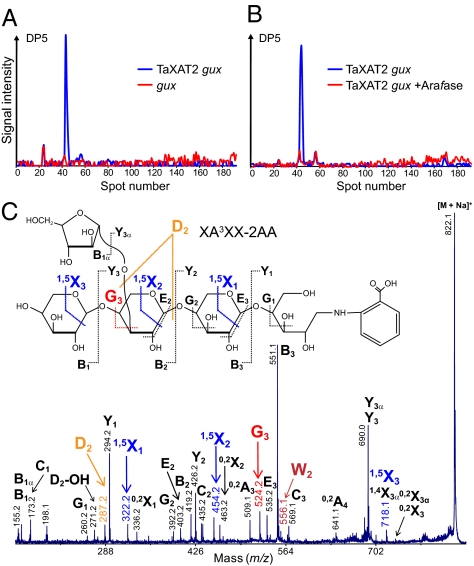
To determine definitively the structure of the XAT-dependent xylan oligosaccharide, we used a modified oligosaccharide relative quantitation using isotope tagging (OliQuIT) method, which allows comparison of oligosaccharide abundances between samples by stable isotope tagging (16). Xylanase-digested AIR from TaXAT2-expressing gux transgenic plants was first compared with oligosaccharides similarly released from gux AIR, and second to TaXAT2-expressing gux transgenic material additionally digested with the arabinofuranosidase. Analysis by capillary normal-phase liquid chromatography (NP-LC) followed by MALDI-ToF-MS shows a single pentose oligosaccharide of DP5 in the XAT transgenic sample (Fig. 3 A and B). In contrast, structures of DP5 were not observed in the untransformed gux sample (Fig. 3A) or the XAT transgenic sample after arabinofuranosidase treatment (Fig. 3B), demonstrating that DP5 is both XAT-dependent and arabinofuranosidase-sensitive. The series of cross-ring 1,5X ions of the MALDI-ToF/ToF-MS/MS analysis showed that a pentose substitution is present on the penultimate xylosyl residue from the reducing end (Fig. 3C). The G3 and D2 elimination ions and the formation of the sugar lactone ion W2 show the presence of a pentose at the C-3 position of this Xylp (Fig. 3C). Taken together with the arabinofuranosidase-sensitivity of the DP5 fragment, it can be unambiguously assigned as XA3XX.
Fig. 3.
Structural analysis of the arabinofuranosidase-sensitive xylan oligosaccharide derived from TaXAT2 gux. (A and B) Extracted ion chromatogram (EIC) of the sodiated molecule DP5 after xylanase digest. Capillary NP-HPLC-MALDI-ToF-MS of AX oligosaccharide labeled with stable isotopes of aniline. The EICs of pentose DP5 labeled with 13C6-aniline (m/z 784) corresponds to TaXAT2 gux (blue) in comparison with labeling with 12C6-aniline (m/z 778; red) of (A) gux and (B) TaXAT2 gux after arabinofuranosidase digest (TaXAT2 gux +Arafase). (C) NP-HPLC-MALDI-ToF/ToF-MS/MS of the DP5 pentose XA3XX labeled with 2-AA (see Fig. S3 and ref. 25 for nomenclature). Ions mentioned in the text are highlighted in color. Note: G3, D2, and W2 ions show a α-(1,3)-linked pentose on the penultimate xylosyl residue.
1D 1H, 2D TOCSY and 13C HSQC NMR of extracted xylan from these plants confirmed that Araf α-(1,3)–linked to monosubstituted Xylp was present in the TaXAT2-transformed gux plants, but was not detected in untransformed gux plants (Fig. S4C). The signal strength suggests that the Ara substitution is present on ∼1% of the xylosyl residues, consistent with the low intensity of the oligosaccharide band by PACE.
To determine whether the TaXAT2-activity is affected by glucuronic acid modifications on the xylan backbone and whether this function is conserved in other grass GT61 enzymes, we analyzed the xylan structure of further GT61-expressing transgenic lines. TaXAT2-expressing transgenic lines in the wild-type background also showed an additional xylan oligosaccharide not present in wild-type (Fig. 2B), and this oligosaccharide was arabinofuranosidase-sensitive (Fig. S5A). Tandem MS (Fig. S5B), together with knowledge of the xylanase and arabinofuranosidase specificities, identified the XAT-dependent oligosaccharide as XU4m2XXA3XX. We did not detect activity of TaXAT1 expressed in wild-type or gux backgrounds. However, we also analyzed FOX lines that express in Arabidopsis the cDNAs of two rice homologs of TaXAT2, OsXAT2 and OsXAT3, under the CaMV 35S-promoter (17). The PACE fingerprint and MS analysis of both these lines also showed the presence of XU4m2XXA3XX (Fig. S5 A and C), and therefore indicates that these rice GT61 enzymes similarly direct the addition of α-(1,3)–linked Araf to the xylan backbone.
Discussion
As shown by RNAi suppression, TaXAT1 is responsible for the majority of monosubstitution of wheat starchy endosperm AX with α-(1,3)–linked Araf (Fig. 1). These results strongly suggest that grass XATs in GT61 Clade A possess xylan α-(1,3)-arabinosyltransferase activity, and this was confirmed by the heterologous expression of wheat and rice XAT genes in Arabidopsis. This activity is quite distinct from the previously described β-(1,2)-xylosyltransferase activity of N-linked glycan synthesis in GT61 Clade C.
We found no evidence that α-(1,2)-, α-(1,3)-arabinosyl di-substitution is dependent on TaXAT1 activity, but the gene function was not entirely lost in the RNAi wheat lines. Therefore, the residual XAT activity could be sufficient to maintain α-(1,3)-arabinosyl substitution in disubstituted Xyl residues. On the other hand, the presence of the yet-uncharacterized GT61 Clade B enzymes and the multiplication of grass GT61s in Clade A might suggest distinct but related proteins are involved in α-(1,2)– and α-(1,3)–substitution of disubstituted Xyl residues. Indeed, the multiplicity of GT61 enzymes would permit a model where each enzyme requires subtly different substrates for arabinosylation, such as xylan already substituted with arabinosyl residues or acetyl groups. This finding could explain why the rice enzymes closely related to TaXAT2 were able to produce identical structure GAX oligosaccharides in transgenic Arabidopsis, whereas products of TaXAT1 were not detected.
Members of the GT61 Clade A are also present in dicots. Expression levels of the Arabidopsis representatives, At3g18180 and At3g18170, are low and they are not coexpressed with secondary cell wall genes (Fig. S6). We found no evidence for arabinosylation of xylan in secondary walls in the absence of TaXAT2 expression. This finding suggests that Arabidopsis GT61s are involved in α-(1,3)-arabinosylation of primary cell wall xylan. Although arabinosylation of xylan has yet to be demonstrated in Arabidopsis, it has been documented in seed mucilage (18) or primary walls (19) of other dicots.
The reduction in Araf α-(1,3)–linked monosubstitution did not cause a concomitant increase in unsubstituted Xylp. This finding suggests there may be a requirement for substitution for continued xylan backbone synthesis in the wheat endosperm, which is unlike the gux xylan synthesis mutants of Arabidopsis, where xylan backbone synthesis is unaffected by the loss of GlcA substitution (7). Unlike acetylated GX synthesis in Arabidopsis, wheat endosperm xylan lacks acetyl substitution. Consequently, unsubstituted regions of xylan are likely to be insoluble. Indeed, the removal of monosubstitution is sufficient to cause a profound decrease in AX solubility presumably because of the greater hydrogen bonding between regions of AX chains lacking any substitution. Because feruloylation occurs via Araf α-(1,3)–linked to monosubstituted Xylp, a requirement of GT61 gene function for the feruloylation, and therefore cross-linking of xylan in grass cell walls, seems probable. Wheat endosperm has exceptionally low feruloylation of AX and GT61 involvement is best investigated in the future in tissues with substantial feruloylation.
Changing the biosynthesis machinery that is required for the decoration of xylan by introducing genes characteristic for grasses into Arabidopsis provides a powerful approach for understanding gene function. The results also indicate potential applications; neither the Arabidopsis transgenics expressing wheat TaXAT2 in gux mutants (where we began to convert the usual dicot GX to AX) nor the wheat transgenics with drastically altered arabinosylation and solubility of AX, showed obvious effects on growth or development. This finding demonstrates the plasticity of xylan structure in planta. We have demonstrated a profound alteration of the properties of AX by manipulation of TaXAT1 in wheat flour, changing soluble fiber to insoluble fiber, so manipulation of GT61 activity promises to allow tailoring of AX biosynthesis to specific end-uses of cereal grain. Manipulation of GT61 genes could also provide a route to improve the digestibility of the vast quantities of lignocellulosic material from grass crops available for biofuel and animal nutrition.
Materials and Methods
Details of plant material, reagents, PCR primers, quantitative RT-PCR, and localization experiments are described in SI Materials and Methods and Table S3.
Phylogenetic Analysis.
Substitutions and indels in GT61 coding DNA sequences were modeled with a maximum likelihood approach to generate the phylogenetic tree. Details are given in SI Materials and Methods.
Construct Preparation and Generation of Transgenic Material.
The TaXAT2 and TaXAT1 full-length cDNA molecules were cloned from wheat var. Cadenza endosperm cDNA and sequenced (European Molecular Biology Laboratory accessions nos. FR873610.1 and FR846232.1, respectively). The TaXAT1 RNAi construct with the starchy endosperm-specific HMW1Dx5 promoter was created, wheat plants transformed and zygosity tested as described in ref. 20 and SI Materials and Methods. Homozygous and azygous (null segregants, used as within-line controls) T2 plants from five independent lines were grown in a statistical design and white flour samples were derived from mature T3 seed, as described in SI Materials and Methods.
The pIRX9::TaXAT2-myc construct was comprised of intergenic regions from IRX9 to drive expression, the TaXAT2 coding sequence and Myc tag. Construct preparation, Arabidopsis transformation and Western blotting analysis are described in SI Materials and Methods.
Analyses of Wheat Endosperm Cell Wall Composition.
Preparation of endosperm cell-wall samples, enzymic digestion, and HPAEC analysis of resulting oligosaccharides were carried out following refs. 21 and 22, with modifications described in SI Materials and Methods. The amount of AX solubilized by this digestion (21) was estimated by monosaccharide analysis as described in ref. 23. Nonstarch polysaccharide from endosperm samples was prepared as described ref. 23 and used for monosaccharide and 1H-NMR analyses, as described in ref. 23 and SI Materials and Methods, respectively.
Analyses of Arabidopsis Xylan.
AIR was prepared, digested, and analyzed by PACE, as described in SI Materials and Methods. Determination of xylan structure was performed by 1H-NMR as described in SI Materials and Methods and a modified OliQuIT method and NP-LC–MALDI-ToF/ToF-MS/MS as follows. Five hundred micrograms to 1 mg AIR was alkaline-treated and digested as described in SI Materials and Methods and then dialyzed using Spectra/Por Biotech CE dialysis membrane MWC: 100–500D (Spectrum Laboratories) to remove excess of smaller fragments. For capillary NP-LC analysis using stable isotope tagging, dried-down samples were reductively aminated with light (12C6) or heavy (13C6) isotopes of aniline. Equal amounts of the differentially labeled samples were mixed, purified and analyzed by NP-LC coupled offline to MALDI-ToF-MS, as previously described (16). This modified OliQuIT method ensures that the chromatographic elution positions on the same oligosaccharides labeled with different isotopes are identical, allowing accurate sample comparison. For structural analysis, samples were reductively aminated with 2-aminobenzoic acid (2-AA), purified and analyzed by NP-LC–MALDI-ToF/ToF-MS/MS, as previously described (24).
Supplementary Material
Acknowledgments
We thank Harry Gilbert and David Bolam for the gift of glycosylhydrolases and Steve Powers for statistical analyses. This work was supported by Biotechnology and Biological Sciences Research Council of the United Kingdom (BBSRC) Grant BB/F014295/1 (to R.A.C.M., P.R.S., and P.D.); and T.T. and J.C.M. were supported by BBSRC Sustainable Bioenergy Centre Cell Wall Sugars Programme Grant BB/G016240/1 (to P.D.). Rothamsted Research receives grant-aided support from the BBSRC. The research leading to these results received funding from European Community's Seventh Framework Programme FP7/2007–2013 under Grant Agreement 211982 (RENEWALL).
Footnotes
Conflict of interest statement: The authors declare that a related patent application has been filed.
Data deposition: The sequences reported in this paper have been deposited in the European Molecular Biology Laboratory database (accession nos. FR873610.1 and FR846232.1).
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1115858109/-/DCSupplemental.
References
- 1.Carpita NC. Structure and biogenesis of the cell walls of grasses. Annu Rev Plant Physiol Plant Mol Biol. 1996;47:445–476. doi: 10.1146/annurev.arplant.47.1.445. [DOI] [PubMed] [Google Scholar]
- 2.Scheller HV, Ulvskov P. Hemicelluloses. Annu Rev Plant Biol. 2010;61:263–289. doi: 10.1146/annurev-arplant-042809-112315. [DOI] [PubMed] [Google Scholar]
- 3.Ishii T. Isolation and characterization of a diferuloyl arabinoxylan hexasaccharide from bamboo shoot cell-walls. Carbohydr Res. 1991;219:15–22. doi: 10.1016/0008-6215(91)89039-i. [DOI] [PubMed] [Google Scholar]
- 4.Sun R, Sun XF, Tomkinson J. Hemicelluloses: Science and Technology. Vol 864. Washington, DC: American Chemical Society; 2003. Hemicelluloses and their derivatives; pp. 2–22. ACS Symposium Series. [Google Scholar]
- 5.Stone B, Morell MK. Carboyhdrates. In: Khan K, Shewry PR, editors. Wheat Chemistry and Technology. St Paul, Minnesota: AACC; 2009. pp. 299–362. [Google Scholar]
- 6.Topping D. Cereal complex carbohydrates and their contribution to human health. J Cereal Sci. 2007;46:220–229. [Google Scholar]
- 7.Mortimer JC, et al. Absence of branches from xylan in Arabidopsis gux mutants reveals potential for simplification of lignocellulosic biomass. Proc Natl Acad Sci USA. 2010;107:17409–17414. doi: 10.1073/pnas.1005456107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mitchell RA, Dupree P, Shewry PR. A novel bioinformatics approach identifies candidate genes for the synthesis and feruloylation of arabinoxylan. Plant Physiol. 2007;144:43–53. doi: 10.1104/pp.106.094995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bencúr P, et al. Arabidopsis thaliana beta1,2-xylosyltransferase: An unusual glycosyltransferase with the potential to act at multiple stages of the plant N-glycosylation pathway. Biochem J. 2005;388:515–525. doi: 10.1042/BJ20042091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Toole GA, et al. Temporal and spatial changes in cell wall composition in developing grains of wheat cv. Hereward. Planta. 2010;232:677–689. doi: 10.1007/s00425-010-1199-5. [DOI] [PubMed] [Google Scholar]
- 11.Porchia AC, Sørensen SO, Scheller HV. Arabinoxylan biosynthesis in wheat. Characterization of arabinosyltransferase activity in Golgi membranes. Plant Physiol. 2002;130:432–441. doi: 10.1104/pp.003400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zeng W, Chatterjee M, Faik A. UDP-Xylose-stimulated glucuronyltransferase activity in wheat microsomal membranes: Characterization and role in glucurono(arabino)xylan biosynthesis. Plant Physiol. 2008;147:78–91. doi: 10.1104/pp.107.115576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baldwin TC, Handford MG, Yuseff MI, Orellana A, Dupree P. Identification and characterization of GONST1, a golgi-localized GDP-mannose transporter in Arabidopsis. Plant Cell. 2001;13:2283–2295. doi: 10.1105/tpc.010247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pellny TK, et al. Cell walls of developing wheat (Triticum aestivum L.) starchy endosperm: Comparison of composition and RNA Seq transcriptome. Plant Physiol. 2011 doi: 10.1104/pp.111.189191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beylot MH, McKie VA, Voragen AG, Doeswijk-Voragen CH, Gilbert HJ. The Pseudomonas cellulosa glycoside hydrolase family 51 arabinofuranosidase exhibits wide substrate specificity. Biochem J. 2001;358:607–614. doi: 10.1042/0264-6021:3580607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ridlova G, Mortimer JC, Maslen SL, Dupree P, Stephens E. Oligosaccharide relative quantitation using isotope tagging and normal-phase liquid chromatography/mass spectrometry. Rapid Commun Mass Spectrom. 2008;22:2723–2730. doi: 10.1002/rcm.3665. [DOI] [PubMed] [Google Scholar]
- 17.Kondou Y, et al. Systematic approaches to using the FOX hunting system to identify useful rice genes. Plant J. 2009;57:883–894. doi: 10.1111/j.1365-313X.2008.03733.x. [DOI] [PubMed] [Google Scholar]
- 18.Fischer MH, et al. The gel-forming polysaccharide of psyllium husk (Plantago ovata Forsk) Carbohydr Res. 2004;339:2009–2017. doi: 10.1016/j.carres.2004.05.023. [DOI] [PubMed] [Google Scholar]
- 19.Darvill JE, Mcneil M, Darvill AG, Albersheim P. Structure of plant-cell Walls. 11. Glucuronoarabinoxylan, a 2nd hemicellulose in the primary-cell walls of suspension-cultured sycamore cells. Plant Physiol. 1980;66:1135–1139. doi: 10.1104/pp.66.6.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nemeth C, et al. Down-regulation of the CSLF6 gene results in decreased (1,3;1,4)-beta-D-glucan in endosperm of wheat. Plant Physiol. 2010;152:1209–1218. doi: 10.1104/pp.109.151712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ordaz-Ortiz JJ, Devaux MF, Saulnier L. Classification of wheat varieties based on structural features of arabinoxylans as revealed by endoxylanase treatment of flour and grain. J Agric Food Chem. 2005;53:8349–8356. doi: 10.1021/jf050755v. [DOI] [PubMed] [Google Scholar]
- 22.Saulnier L, et al. Wheat endosperm cell walls: Spatial heterogeneity of polysaccharide structure and composition using micro-scale enzymatic fingerprinting and FT-IR microspectroscopy. J Cereal Sci. 2009;50:312–317. [Google Scholar]
- 23.Englyst HN, Quigley ME, Hudson GJ. Determination of dietary fibre as non-starch polysaccharides with gas-liquid chromatographic, high-performance liquid chromatographic or spectrophotometric measurement of constituent sugars. Analyst (Lond) 1994;119:1497–1509. doi: 10.1039/an9941901497. [DOI] [PubMed] [Google Scholar]
- 24.Maslen SL, Goubet F, Adam A, Dupree P, Stephens E. Structure elucidation of arabinoxylan isomers by normal phase HPLC-MALDI-TOF/TOF-MS/MS. Carbohydr Res. 2007;342:724–735. doi: 10.1016/j.carres.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 25.Domon B, Costello CE. A systematic nomenclature for carbohydrate fragmentations in Fab-Ms Ms spectra of glycoconjugates. Glycoconj J. 1988;5:397–409. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.