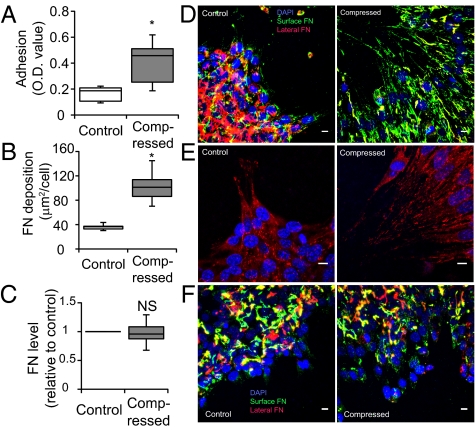
Fig. 5.
Compression up-regulates 67NR cell–matrix adhesion via localized fibronectin secretion. (A) Compression enhances cell-substrate adhesion. Uncompressed and compressed samples were exposed to detractive fluid shear and the remaining adherent cells were quantified using a colorimetric assay in which crystal violet stain was quantified via optical density (OD) at 540 nm (n = 8; *P < 0.005). (B) Quantification of fibronectin accumulation at the cell–substrate interface. Results are expressed as surface fibronectin-positive pixel area relative to the total number of DAPI-stained nuclei (n = 11–12; *P < 0.005 compared with the control). (C) Quantitative PCR of control and compressed 67NR cells showed no significant difference in fibronectin messenger level between the two groups (NS, not significant). (D) Fibronectin staining of 67NR cells at the periphery of the cell-denuded area. Fibronectin at the cell–substrate interface in the compressed, but not control, samples was fibrillar and oriented in the direction of migration (n = 17). (Scale bar, 10 μm.) (E) Vinculin-stained cells at the periphery of the cell-denuded area. 67NR cells were either uncompressed (control) or exposed to a compressive stress of 5.8 mmHg for 16 h. Vinculin-positive (red) focal adhesions were detected underneath compression-induced filopodia of elongated cells (n = 16). (Scale bar, 10 μm.) (F) Fibronectin staining of 67NR cells treated with 1 μM cycloheximide at the periphery of the cell-denuded area. The formation of oriented and fibril-like patterns of fibronectin observed earlier in the nontreated compressed cultures (D) was abolished after inhibition of protein synthesis, suggesting that the cells secrete fibronectin during their movement for enhanced cell–matrix adhesion (n = 8). (Scale bar, 10 μm.)