Abstract
The benefits of bioluminescence for nonsymbiotic marine bacteria have not been elucidated fully. One of the most commonly cited explanations, proposed more than 30 y ago, is that bioluminescence augments the propagation and dispersal of bacteria by attracting fish to consume the luminous material. This hypothesis, based mostly on the prevalence of luminous bacteria in fish guts, has not been tested experimentally. Here we show that zooplankton that contacts and feeds on the luminescent bacterium Photobacterium leiognathi starts to glow, and demonstrate by video recordings that glowing individuals are highly vulnerable to predation by nocturnal fish. Glowing bacteria thereby are transferred to the nutritious guts of fish and zooplankton, where they survive digestion and gain effective means for growth and dispersal. Using bioluminescence as bait appears to be highly beneficial for marine bacteria, especially in food-deprived environments of the deep sea.
Bioluminescence is common in the marine environment, occurring in numerous organisms, from bacteria to invertebrates and fish (1, 2). Bacterial bioluminescence occurs as a continuous glow in the presence of oxygen at cell concentrations exceeding quorum-sensing levels (3–6). Luminous bacteria occur free-living in seawater (7, 8), in symbiotic associations with marine organisms (most notably fish and squids; see refs. 7 and 8 and references therein), as saprophytes on suspended organic material such as marine snow (9, 10), as a major component of fecal pellets (11–13), and as parasites on crustaceans (14).
Although the adaptive benefits of energetically costly bioluminescence in symbiotic bacteria are well understood (e.g., 7, 15), those benefits in nonsymbiotic bacteria and those living as ectoparasites on zooplankton are less obvious. Several different physiological and biochemical functions of bacterial bioluminescence have been proposed (7, 16–20), focusing mostly on antioxidative activity, enhanced DNA repair, and UV resistance, although the validity of some of these hypotheses has been questioned (21).
An ecological function in propagation and dispersal also has been postulated (6, 7, 22). According to this hypothesis (hereafter, “bait hypothesis“), the bacteria, by glowing, visually mark the presence of a food particle for fish in order to get into their nutritious guts. So far, this hypothesis was supported by circumstantial evidence showing that luminous bacteria thrive in and survive passage through fish guts (7, 12, 23, 24). Here we propose that the mechanism underlying the bait hypothesis is based on the following steps: (i) Quorum sensing assures that bacterial bioluminescence is a reliable signal of the presence of food aggregates, e.g., marine snow; (ii) zooplankton is attracted to luminous particles and grazes on the bacteria-rich organic matter; (iii) because of its contact with or ingestion of the luminous bacteria, the zooplankton itself becomes glowing; (iv) the glowing zooplankton is detected readily and consumed by fish; (v) once in the gut of either zooplankton or fish, the bacteria gain a nutritious environment for growth and a fast-moving vehicle for wide dispersal. The scheme may be shortened by fish that directly detect and consume glowing organic particles without zooplankton being involved, or by zooplankton that propagates bacteria in its feces.
The objective of this study was to test the following key points of the bait hypothesis: (i) visual attraction of zooplankton to bacterial bioluminescence; (ii) promotion of glow in zooplankton contacting/ingesting luminous bacteria (using planktonic brine shrimps as a surrogate for zooplankton); (iii) attraction of zooplanktivorous fish to glowing prey; and (iv) survival by bacteria of gut passage in both zooplankton and fish.
Results
Zooplankton Attraction to Bacterial Bioluminescence.
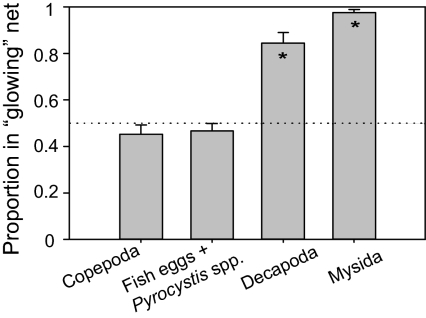
A large (135-L) experimental sea-water tank was used to examine whether the luminescence of marine bacteria (Photobacterium leiognathi) attracts zooplankton. A dialysis bag (20 mL) containing luminous bacteria, used as a bait, was placed at one corner of the tank; an identical bag containing a culture of a dark mutant of P. leiognathi was placed at the opposite corner. Significant changes in zooplankton distribution within the tank were noticeable within 15 min. Decapods and mysids were found almost exclusively (one-sample t-test, P < 0.001 for both decapods and mysids) over the glowing net, whereas copepods showed no significant attraction (P = 0.269) to either net (Fig. 1). Similarly, no difference was found for nonmotile organisms (spherical fish eggs and Pyrocystis spp.; P = 0.34), which served as in internal control. Together these four groups constituted on average 85% (range, 73–90%) of the plankton captured in our samples. Other taxonomic groups were too rare to be included reliably in this comparison.
Fig. 1.
Zooplankton attraction to the bioluminescence of P. leiognathi shown as average proportions (total in glowing net divided by totals in both nets + SEM; n = 8) of four selected zooplankton taxonomic groups. Asterisks indicate a significant difference (one-sample t test, P < 0.001 for each taxon) from the value of 0.5 expected under no attraction to either net (dotted line).
Zooplankton Turns Luminescent upon Contacting P. leiognathi Cultures.
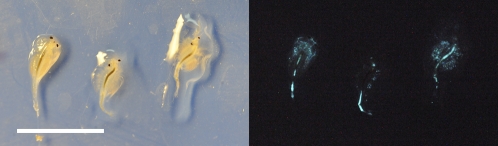
The brine shrimp Artemia salina (hereafter “Artemia”) became luminescent after swimming for only 10 s in a liquid culture of P. leiognathi, as well as after swimming for 2.5 h in the suspension of small particles of bioluminescent P. leiognathi colonies. As revealed by long-exposure photographs, the luminescence in the guts of Artemia was clearly visible from outside, with additional glow produced by bacteria attached externally to the exoskeleton and appendages (Fig. 2). Similarly, nonglowing individual marine mysids, Anisomysis marisrubri, freshly trapped in the sea, started to glow after contacting a diluted culture of P. leiognathi.
Fig. 2.
Glow of zooplankton (A. salina) after contacting and ingesting small particles broken off colonies of the bioluminescent bacterium P. leiognathi. The photograph on the left was taken in room light, and the photograph on the right was taken in darkness using long exposure (30 s) with a Nikon D3 camera (f/5.6, ISO 25600, 150 mm lens). (Scale bar: 1 cm.)
Fish Detect and Consume Glowing Prey.
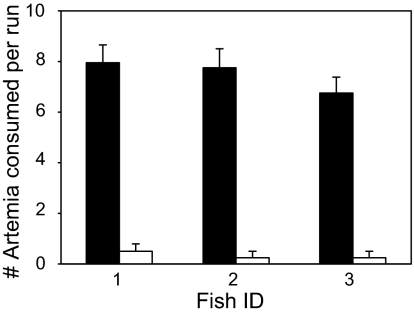
The promoted glow in Artemia dramatically affected its risk of being preyed on by the nocturnal fish Apogon annularis in a recirculating laboratory flume in the dark. Almost all the glowing Artemia offered to the fish were consumed readily (Fig. 3 and Movie S1), compared with rare occasions of predation on nonglowing specimens. As the video recordings revealed, the predation on nonglowing specimens occurred only when the prey drifted by chance directly toward the fish's head. The effect of glow of the prey on fish predation was highly significant (two-way ANOVA, F1,18 = 275.648, P < 0.0001), whereas the effect of the identity of the fish was not significant (two-way ANOVA, F2,18 = 0.990, P = 0.39). Analysis of the video recordings revealed that the fish actively attacked and consumed glowing prey (Movie S1), whereas the nonglowing prey passed undetected even at very close proximity to the fish (Movie S2). Glowing prey were detected by the fish from a distance of up to 26.8 cm, near the limit allowed by the working section of the flume. In fact, video records revealed that the fish occasionally swam to the upstream part of the working section and seemed to wait for the prey to arrive. This behavior suggests that the fish detected the prey even before it entered its feeding chamber (at a distance >30 cm).
Fig. 3.
The average (+SEM) number of glowing (black bars) and nonglowing (open bars) Artemia captured by the nocturnal coral-reef fish A. annularis per run (n = 4) in a recirculating flume in complete darkness. Each run lasted 2.5 min. The effect of prey glow on fish predation was highly significant (two-way ANOVA, F1,18 = 275.648, P < 0.0001), whereas the effect of fish identity was not significant (two-way ANOVA, F2,18 = 0.990, P = 0.39).
Bacteria Survive and Luminesce Strongly Following Passage Through Zooplankton and Fish Guts.
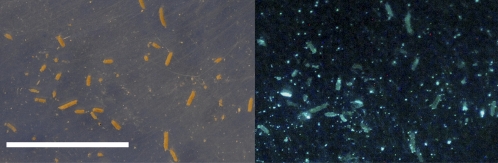
Glow was detected clearly in the fecal pellets of Artemia that fed on detached colony particles of luminous P. leiognathi (Fig. 4), indicating that the luminescent bacteria survived the passage through the guts. Similarly, viable luminescent P. leiognathi cells were found in the fecal pellets passed by the marine mysid A. marisrubri after the mysids were allowed to feed in a diluted culture of the bacterium. The average number ± SE of luminous cfu per fecal pellet in these pellets (“second-step” pellets; Materials and Methods) was 1,100 ± 183, several orders of magnitude higher than in the pellets collected from the same mysid individuals before exposure to the bacterial culture (“first-step” pellets) and control (“third-step”) pellets (0.05 ± 0.03 and 21 ±14 respectively; paired t test, P < 0.005, for details see Materials and Methods).
Fig. 4.
Fecal pellets of A. salina produced after swimming and feeding on small colony fragments of the bioluminescent bacterium P. leiognathi (visible in the background) photographed in room light (Left) and in darkness (Right) as in Fig. 2. (Scale bar: 1 cm.)
High concentrations of viable P. leiognathi were found also in the feces of the fish A. annularis that had fed on luminous Artemia. The abundance of luminous cells in these feces was five orders of magnitude higher than in the feces of the fish that had fed on nonluminous Artemia (1.35 × 105 vs. <10 cfu in the tested feces samples, respectively).
Discussion
Our study provides experimental evidence for some key steps of the bait hypothesis, elucidating the benefits of bioluminescence in marine nonsymbiotic bacteria. According to this hypothesis, zooplankton is attracted to luminous particles rich in organic food; because luminescence is quorum-dependent, particles that are poor in available organics are unlikely to sustain a sufficiently high density of bacteria to generate luminescence (the quorum-sensing threshold of P. leiognathi is ∼108⋅mL−1; Fig. S1).
The next prediction of the bait hypothesis is that, on contact with and ingestion of luminous particles, the zooplankton itself starts to glow, thereby attracting its own predators, such as fish. The advantage to the bacteria is obvious: By surviving digestion in the guts of both zooplankton and fish, the bacteria gain a nutrient-rich, sheltered environment for proliferation as well as an efficient means of dispersal. The advantage for zooplankton is less obvious because of the tradeoff between the gain provided by organic-rich food and the cost incurred by a higher risk of predation.
To examine the bait hypothesis, we experimentally tested its key steps. First, we documented the visual attraction of marine zooplankton to bacterial bioluminescence (Fig. 1). Biolumines-cence in most marine bacteria peaks at the wavelength of ∼490 nm (25), which, not surprisingly, is near the wavelength least absorbed in seawater (26). Several zooplankton taxa [e.g., two species of the copepod Pleuromamma (27), hyperiid amphi-pods (28), and some deep-sea crustaceans (29)] were shown to be sensitive to similar wavelengths. Mesopelagic crustaceans have a single peak of spectral sensitivity at 470–500 nm, presumably exhibiting greater sensitivity to bioluminescence than to downwelling light (30). Bacterial bioluminescence might be used by zooplankton as a visual cue during its search for food; such a cue is likely to be detectable in the dark at much greater distances than chemical or mechanical cues.
Our laboratory experiments also corroborated the second step of the bait hypothesis, that contacting or ingesting luminous bacteria imparts sustained glow in the zooplankter (Fig. 2 and Fig. S2). Although our experiment did not differentiate between the glow generated by externally attached bacteria and those ingested (Fig. 2), both sources are likely to occur in nature and are not mutually exclusive. Artemia was used in this study as a surrogate for actual zooplankton prey because it is readily eaten by zooplanktivorous fishes (31–35), is easy to handle, and lacks evasive behavior. The third characteristic was expected to reduce the variability of successful predation by the fish, allowing better separation of the effect of luminescence in our predation experiment. The potential bias of our reliance on a nonmarine zooplankton, Artemia, is alleviated by the findings that the marine mysid A. marisrubri also glows after contacting bioluminescent P. leiognathi, and that the intensity of its glow is similar to that of the glowing Artemia. Although A. marisrubri resides in shallow waters (36), we assume that the mechanism by which these two animals become luminous after contacting P. leiognathi is not different from that of other, open-water, planktonic crustaceans, all having a partly transparent, chitinous exoskeleton and numerous appendages.
The third step of the bait hypothesis is corroborated by our findings that the glow promoted in zooplankters after contacting luminescent bacteria greatly enhances their vulnerability to visual predators in the dark (Fig. 3 and Movies S1 and S2). Contacting the bacteria thus can be risky for the zooplankton. How has such a potentially deleterious characteristic endured natural selection? We propose that the step sequence of the bait mechanism is most applicable for food-deprived environments, such as the deep sea, where the abundance of marine snow decreases with depth (37–39) and food availability becomes a major limiting factor. Because the density dependence of bacterial bioluminescence (3–6) is a reliable indicator of a rich patch of food, the benefit of finding rare food in nutrient-deprived waters may outweigh the increase in predation risk.
Marine snow is consumed by zooplankton (40–43), and fecal pellets also might be used as a food source through coprophagy (44–46). Although our experiments with liquid cultures of P. leiognathi did not fully simulate in situ conditions, the fact that similar glow was promoted in Artemia that ingested particles of the bacterial colonies (Figs. 2 and 4) supports the assertion that glow would be promoted in zooplankton after contacting and ingesting luminous marine snow and fecal pellets in the sea. However, experiments with real marine snow are needed to test this claim explicitly.
High oxygen concentrations are essential for the obligately aerobic process of bioluminescence. Within sinking organic aggregates oxygen concentrations are reported to be >80% of air saturation (47), whereas other studies demonstrate that oxygen-depleted microzones can develop in marine snow aggregates (48, 49). However, luminescence seems to take priority, because it prevails even at concentrations where the growth of the luminescent bacteria is limited by oxygen (6). Furthermore, some strains of luminous bacteria display increased luminescence at growth-limiting oxygen conditions (6).
Some freshwater bacteria are able to “hitchhike” on migrating zooplankton (i.e., actively to associate and dissociate from them), thereby enhancing their dispersal through the water column (50). Our findings indicate that bioluminescence in bacteria is a very effective means to obtain such hitchhikes. The dispersal rate provided by migrating zooplankton, and even more so by actively swimming fish, is several orders of magnitude greater than that of water-borne free bacteria. Although long-distance dispersal can be costly when individuals are dispersed into habitats less favorable than their origin (51), for wide-spread organisms such as luminous bacteria, found over large depth ranges in different oceanic and coastal habitats (8), the likelihood of reaching an unfavorable environment is small. In addition, long-distance dispersal allows exploitation of new ephemeral resources and reduces the risk of species extinction (52). However, the most important advantage of being bait seems to be obtaining a lush substrate for growth and proliferation in the guts (Fig. 4). That luminous bacteria are common in fish guts and survive digestion has been shown in numerous studies (7, 12, 23, 24). Our findings add to these studies the observation that the bacteria maintain their luminescence in the zooplankton guts, where oxygen levels may be lower than in the open water, and that luminescence in the zooplankton guts is detectable from the outside.
In conclusion, our study shows that quorum-dependent bioluminescence in nonsymbiotic bacteria is a visual attractant for zooplankton and fish, which, in turn, provide the ingested bacteria with lush substrate for proliferation and effective means for dispersal.
Materials and Methods
Bacterial Cultures.
The luminous bacterium used in this study was P. leiognathi strain MI1 (SI Materials and Methods) isolated from a depth of 600 m in the Gulf of Aqaba, Red Sea, and identified by 16 S rRNA sequencing using the universal Eubacterial 27-f and 1492-r primers set (53). To obtain a dark mutant, the wild-type strain was mutagenized with N-methyl-N'-nitro-N-nitrosoguanidine (54). Clones that failed to glow in the dark were isolated, their taxonomic identity was verified by 16 S rRNA sequencing as described above, and one of these clones (strain MI1d) was selected for use in this study. Bioluminescence of bacterial cultures was verified by visual inspection in a dark room and by measuring the relative luminescence of 1 mL of culture using a custom-made luminometer (Model 597D; Technion). Luminescence intensity of the cultures used in feeding experiments (see below) varied between 8 × 1010 and 1.5 × 1011 photons⋅s−1⋅mL bacterial culture. Cultures of the dark mutant exhibited no measurable light emission.
Zooplankton Attraction to Bacterial Luminescence.
Attraction of zooplankton to bacterial luminescence was examined in vitro at the pier of the Interuniversity Institute for Marine Sciences (IUI), the Gulf of Aqaba, Red Sea. An opaque container (0.75 m × 0.55 m × 0.4 m) was filled with freshly collected seawater. Two plankton hand nets (25-cm mouth diameter, 200-μm mesh size) were placed on the bottom of the container in opposite corners. The nets were tied to a long horizontal rod that allowed their lifting out of the water simultaneously, thereby collecting the zooplankton swimming above each net. Dialysis bags (SnakeSkin pleated dialysis tubing; Thermo Scientific) filled with 20 mL of either a bioluminescent or a dark-mutant culture of P. leiognathi were placed at the center of the two nets (hereafter “glowing” and “dark” nets, respectively), with their ends connected to small weights serving as anchors. The positions of the bioluminescent bait and its dark control were switched between the two nets in consecutive runs.
Starting 0.5–1 h after sunset, zooplankton was sampled by two swimmers horizontally towing a plankton net (50-cm mouth diameter, 300-μm mesh size) close to the sea surface for 15–20 min. The collected plankton was transferred gently to a 1-L jar and afterward was introduced slowly into the experimental tank through a plastic pipe held at the center of the tank to minimize turbulence-related plankton dispersal in the tank. The tank then was covered with a light-tight cover, and the introduced zooplankton was allowed to swim in the tank undisturbed for 15 min.
After 15 min the two hand nets were lifted simultaneously. The animals trapped in each hand net were preserved in 4% buffered formalin and transferred to the laboratory for later counting under a dissecting microscope. The experiment was carried out eight times, twice each night for four nights between November 9, 2009, and November 24, 2009. The number of individuals in each taxonomic group in the glowing net was divided by the corresponding total trapped in both nets, yielding a proportion value between 0 and 1, indicating complete avoidance or complete attraction to bacterial luminescence, respectively. A value of 0.5 was expected for taxa that do not respond to the bacterial luminescence.
Production of Glowing Prey.
Prey used in the fish-feeding experiment (see below) were live, adult brine shrimps A. salina (“Artemia”), ∼1 cm in length, with no egg sac. Fresh specimens were collected every few days and kept unfed in aerated aquaria filled with filtered sea water (FSW, filtered through 0.7-μm Whatman glass microfibre filters). Before each fish-feeding run (see below), about 15 adult Artemia were placed for 10 s in a liquid culture of bioluminescent P. leiognathi, thoroughly rinsed with FSW in a sieve with a mesh size of 55 μm, and allowed to swim freely for 5 min in 500 mL of FSW. After the presence of glow was verified in each individual, a batch of 10 glowing Artemia was separated for later use in the fish-predation experiment. Control experiments used an identical protocol with the bacterium's dark mutant. Repetitive luminescence measurements of the same batch of glowing Artemia (n = 5 batches) indicated that detectable glow persisted for several hours, declining in the first 20 min to 37% of the initial value and declining further to 16% of the initial value after 2 h (Fig. S2). Artemia, a nonmarine animal, was used because of its availability in large quantities of similar size and its excellent tolerance of the laboratory handling. A complementary test of glow promotion in a marine zooplankter was carried out with the mysid A. marisrubri, found in the shallow waters off IUI. Ten adult mysids (∼1 cm in length) were separated from a plankton hand net, allowed to swim for 1 min in a diluted bioluminescent bacterial culture, briefly washed with FSW, and allowed to swim in FSW for 5 min. Their luminescence was measured in the luminometer.
Because luminous bacteria in the ocean often occur on organic particles such as marine snow and fecal pellets, we also tested whether the ingestion of small luminous particles would induce a glow in Artemia. Here the animals were allowed to graze for 2.5 h on minute pieces of bioluminescent P. leiognathi colonies suspended in a beaker filled with FSW (SI Materials and Methods). A strong glow resulted both inside the guts (visible from outside) and on the external body surface (Fig. 2).
Fish-Feeding Experiments.
The effect of glow on detectability by predators was examined with the glowing Artemia as prey and the small (7–10 cm) nocturnal zooplanktivorous fish A. annularis, common in shallow reefs throughout the Red Sea, as predator. The fish is a visual predator with excellent light sensitivity, previously demonstrated to detect nonglowing prey (>1 mm in length) from a distance of up to 20 cm under light conditions equivalent to a few meters depth on a clear, moonless night (33). Relying only on visual cues, the fish is unable to detect prey in complete darkness (33). Therefore, our feeding experiment was carried out in a fully darkened laboratory, using a large laboratory flume (2 m long, 30 × 30 cm in cross-section) (32) operated at a flow speed of 6 cm⋅s−1 (SI Materials and Methods). Feeding rates on glowing and nonglowing Artemia were measured under IR illumination as previously described (33), using an IR-sensitive video camera to record the behavior of the fish.
Each trial consisted of two parts. In each part, 10 glowing or 10 dark (control) Artemia were offered to a single fish in the flume. One or two trials were carried out each night; the order of glowing and control Artemia offerings was randomized. Four complete trials were carried out with each of three individual A. annularis. Satiation was unlikely, because A. annularis can consume >20 adult Artemia (33) continuously, and the maximum number consumed each night in our experiment never exceeded 11. The experiment protocol (SI Materials and Methods) was based on measurements of the total number of Artemia consumed during 2.5 min. The video records were used to measure the reactive distance, defined as the distance between the fish and prey at the time of strike initiation.
Luminous Bacteria in Feces of Fish and Zooplankton.
To examine whether P. leiognathi survives the passage through the digestive tract of A. annularis, a fish which previously had been starved for 24 h was fed with 20 glowing Artemia, washed with FSW, transferred to a 2-L plastic tank filled with FSW, and allowed to defecate overnight. In the morning the feces were washed thoroughly with sterilized FSW, torn apart using a sterilized needle, suspended in 1 mL sterilized FSW, and, after serial dilutions, were spread on seawater-based LB agar plates. The plates were incubated at 30 °C overnight, and the number of luminous cfu was determined. To assess the possibility that luminous bacteria were present initially in the gut of A. annularis, another fish was fed with 20 nonluminous Artemia, and its feces were analyzed similarly.
P. leiognathi's survival of the passage through the digestive system of the marine mysid A. marisrubri was examined similarly in fecal pellets produced by bacteria-fed mysids. Eight mysids, freshly caught by a hand net in the shallow reef in front of the IUI, were allowed to swim and empty their guts in a Petri dish filled with sterile FSW. Their fecal pellets were collected 1.5 h later (hereafter “first-step” pellets). Immediately afterward these mysids were transferred to a dilute culture of luminescent P. leiognathi for 5 min, washed with FSW, allowed to swim in FSW for additional 5 min, and then were placed in sterile FSW, from which their fecal pellets were collected 1.5 h later (hereafter “second-step” pellets). Because the second-step pellets could have been contaminated with P. leiognathi shed from the mysids’ outer skeleton, control “third-step” pellets were produced by immersing some of the first-step pellets for 1.5 h in dishes filled with the water in which the second-step pellets were produced. Care was taken that this water was free of mysids and pellets. Thus second- and third-step pellets were exposed to the same water for the same duration. All pellets were examined for the presence of luminous bacteria using an identical protocol (SI Materials and Methods).
Statistical Analyses.
A paired t test was performed where the comparison was based on paired data (e.g., number of luminous cfu in step 1 and step 2 pellets). Means of bioluminescence intensity in Artemia and mysids were compared using a two-sample t test, whereas a one-sample t test was used to test the difference between the mean proportion of each zooplankton taxon in the “glowing” net and the expected proportion of 0.5. Two-way ANOVA was used to test for the effect of bacteria-promoted luminescence in Artemia and the effect of fish on the predation of A. annularis, after testing for homogeneity of variance using the Cochran test. Statistical analyses were carried out using SYSTAT (version 9 for Windows).
Supplementary Material
Acknowledgments
We are indebted to M. Ohevia, R. Holzman, S. Penno, and S. Weinberger for assistance; to V. China for photography; to the Marine Underwater Observatory for providing Artemia; to the staff of the Interuniversity Institute for Marine Sciences of Eilat for logistic help; and to J. W. Hastings and M. Latz for comments on an earlier draft. This study was funded by an Israel Science Foundation grant (to A.G.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1116683109/-/DCSupplemental.
References
- 1.Widder EA. Bioluminescence in the ocean: Origins of biological, chemical, and ecological diversity. Science. 2010;328:704–708. doi: 10.1126/science.1174269. [DOI] [PubMed] [Google Scholar]
- 2.Haddock SHD, Moline MA, Case JF, et al. Bioluminescence in the sea. Ann Rev Mar Sci. 2010;2:443–493. doi: 10.1146/annurev-marine-120308-081028. [DOI] [PubMed] [Google Scholar]
- 3.Nealson KH, Platt T, Hastings JW. Cellular control of the synthesis and activity of the bacterial luminescent system. J Bacteriol. 1970;104:313–322. doi: 10.1128/jb.104.1.313-322.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eberhard A. Inhibition and activation of bacterial luciferase synthesis. J Bacteriol. 1972;109:1101–1105. doi: 10.1128/jb.109.3.1101-1105.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nealson KH. Autoinduction of bacterial luciferase. Occurrence, mechanism and significance. Arch Microbiol. 1977;112:73–79. doi: 10.1007/BF00446657. [DOI] [PubMed] [Google Scholar]
- 6.Hastings JW, Nealson KH. Bacterial bioluminescence. Annu Rev Microbiol. 1977;31:549–595. doi: 10.1146/annurev.mi.31.100177.003001. [DOI] [PubMed] [Google Scholar]
- 7.Nealson KH, Hastings JW. Bacterial bioluminescence: Its control and ecological significance. Microbiol Rev. 1979;43:496–518. doi: 10.1128/mr.43.4.496-518.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dunlap PV, Kita-Tsukamoto K. In: The Prokaryotes. 3rd Ed. Dworkin M, Falkow S, Rosenberg E, Schleifer K-H, Stackebrandt E, editors. Vol 2. New York: Springer; 2006. pp. 863–892. [Google Scholar]
- 9.Orzech JK, Nealson KH. In: Ocean Optics VII. Blizard M, editor. Vol 489. Bellingham, WA: International Society for Optical Engineering; 1984. pp. 100–106. [Google Scholar]
- 10.Orzech JK. La Jolla, CA: Univ of California at San Diego; 1988. “Marine Snow” as an Emitter of Light in the Sea. PhD dissertation. [Google Scholar]
- 11.Andrews CC, Karl DM, Small LF, Fowler SN. Metabolic activity and bioluminescence of oceanic fecal pellets and sediment trap particles. Nature. 1984;307:539–540. [Google Scholar]
- 12.Ruby EG, Morin JG. Luminous enteric bacteria of marine fishes: A study of their distribution, densities, and dispersion. Appl Environ Microbiol. 1979;38:406–411. doi: 10.1128/aem.38.3.406-411.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raymond JA, DeVries AL. Bioluminescence in McMurdo Sound, Antarctica. Limnol Oceanogr. 1976;21:599–602. [Google Scholar]
- 14.Inman OL. A pathogenic luminous bacterium. Biol Bull. 1926;53:197–200. [Google Scholar]
- 15.Visick KL, Ruby EG. Vibrio fischeri and its host: It takes two to tango. Curr Opin Microbiol. 2006;9:632–638. doi: 10.1016/j.mib.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 16.Rees J-F, et al. The origins of marine bioluminescence: Turning oxygen defence mechanisms into deep-sea communication tools. J Exp Biol. 1998;201:1211–1221. doi: 10.1242/jeb.201.8.1211. [DOI] [PubMed] [Google Scholar]
- 17.Kasai S. Occurrence of P-flavin binding protein in Vibrio fischeri and properties of the protein. J Biochem. 1999;126:307–312. doi: 10.1093/oxfordjournals.jbchem.a022450. [DOI] [PubMed] [Google Scholar]
- 18.Czyz A, Wróbel B, Węgrzyn G. Vibrio harveyi bioluminescence plays a role in stimulation of DNA repair. Microbiology. 2000;146:283–288. doi: 10.1099/00221287-146-2-283. [DOI] [PubMed] [Google Scholar]
- 19.Szpilewska H, Czyz A, Węgrzyn G. Experimental evidence for the physiological role of bacterial luciferase in the protection of cells against oxidative stress. Curr Microbiol. 2003;47:379–382. doi: 10.1007/s00284-002-4024-y. [DOI] [PubMed] [Google Scholar]
- 20.Cutter KL, Alloush HM, Salisbury VC. Stimulation of DNA repair and increased light output in response to UV irradiation in Escherichia coli expressing lux genes. Luminescence. 2007;22:177–181. doi: 10.1002/bio.946. [DOI] [PubMed] [Google Scholar]
- 21.Walker EL, Bose JL, Stabb EV. Photolyase confers resistance to UV light but does not contribute to the symbiotic benefit of bioluminescence in Vibrio fischeri ES114. Appl Environ Microbiol. 2006;72:6600–6606. doi: 10.1128/AEM.01272-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robison BH, Ruby EG, Morin JG. Luminous bacteria associated with the gut contents of midwater fishes. West Soc Nat. 1977;58:55. [Google Scholar]
- 23.O'brien CH, Sizemore RK. Distribution of the luminous bacterium Beneckea harveyi in a semitropical estuarine environment. Appl Environ Microbiol. 1979;38:928–933. doi: 10.1128/aem.38.5.928-933.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Makemson JC, Hermosa GV., Jr Luminous bacteria cultured from fish guts in the Gulf of Oman. Luminescence. 1999;14:161–168. doi: 10.1002/(SICI)1522-7243(199905/06)14:3<161::AID-BIO538>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 25.Nealson KH, Hastings JW. In: The Prokaryotes. Balows A, Trüper HG, Dworkin M, Harder W, Schleifer K-H, editors. Berlin: Springer; 1991. pp. 625–639. [Google Scholar]
- 26.Parsons TR, Takahashi M, Hargrave B. Biological Oceanographic Processes. Oxford, UK: Pergamon; 1984. [Google Scholar]
- 27.Buskey EJ, Baker KS, Smith RC, Swift E. Photosensitivity of the oceanic copepods Pleuromamma gracilis and Pleuromamma xiphias and its relationship to light penetration and daytime depth distribution. Mar Ecol Prog Ser. 1989;55:207–216. [Google Scholar]
- 28.Land MF, Marshall NJ, Diebel C. Tracking of blue lights by hyperiid amphipods. J Mar Biol Assoc U K. 1995;75:71–81. [Google Scholar]
- 29.Frank TM, Case JF. Visual spectral sensitivities of bioluminescent deep-sea crustaceans. Biol Bull. 1988;175:261–273. [Google Scholar]
- 30.Frank TM, Widder EA. Comparative study of the spectral sensitivities of mesopelagic crustaceans. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 1999;185:255–265. [Google Scholar]
- 31.Coughlin DJ, Strickler JR. Zooplankton capture by a coral reef fish: An adaptive response to evasive prey. Environ Biol Fishes. 1990;29:35–42. [Google Scholar]
- 32.Kiflawi M, Genin A. Prey flux manipulation and the feeding rates of reef-dwelling planktivorous fish. Ecology. 1997;78:1062–1077. [Google Scholar]
- 33.Holzman R, Genin A. Zooplanktivory by a nocturnal coral-reef fish: Effects of light, flow, and prey density. Limnol Oceanogr. 2003;48:1367–1375. [Google Scholar]
- 34.Sørnes TA, Aksnes DL. Efficiency in visual and tactile zooplanktivores. Limnol Oceanogr. 2004;49:69–75. [Google Scholar]
- 35.Rickel S, Genin A. Twilight transitions in coral reef fish: The input of light-induced changes in foraging behavior. Anim Behav. 2005;70:133–144. [Google Scholar]
- 36.Echelman T, Fishelson L. Surface zooplankton dynamics and community structure in the Gulf of Aqaba (Eilat), Red Sea. Mar Biol. 1990;107:179–190. [Google Scholar]
- 37.Honjo S, Doherty KW, Agrawal YC, Asper VL. Direct optical assessment of large amorphous aggregates (marine snow) in the deep ocean. Deep-Sea Res. 1984;31:67–76. [Google Scholar]
- 38.Asper VL, Smith WO., Jr Abundance distribution and sinking rates of aggregates in the Ross Sea, Antarctica. Deep-Sea Res. 2003;50:131–150. [Google Scholar]
- 39.Guidi L, et al. Relationship between particle size distribution and flux in the mesopelagic zone. Deep-Sea Res. 2008;55:1364–1374. [Google Scholar]
- 40.Lampitt RS, Wishner KF, Turley CM, Angel V. Marine snow studies in the Northeast Atlantic Ocean: Distribution, composition and role as a food source for migrating plankton. Mar Biol. 1993;116:689–702. [Google Scholar]
- 41.Schnetzer A, Steinberg D. Natural diets of vertically migrating zooplankton in the Sargasso Sea. Mar Biol. 2002;141:89–99. [Google Scholar]
- 42.Dilling L, Brzezinski MA. Quantifying marine snow as a food choice for zooplankton using stable silicon isotope tracers. J Plankton Res. 2004;26:1105–1114. [Google Scholar]
- 43.Koski M, Møller EF, Maar M, Visser AW. The fate of discarded appendicularian houses: Degradation by the copepod, Microsetella norvegica, and other agents. J Plankton Res. 2007;29:641–654. [Google Scholar]
- 44.Paffenhöfer GA, Knowles SC. Ecological implications of fecal pellet size, production and consumption by copepods. J Mar Res. 1979;37:35–49. [Google Scholar]
- 45.Lampitt RS, Noji T, von Bodungen B. What happens to zooplankton fecal pellets? Implications for material flux. Mar Biol. 1990;104:15–23. [Google Scholar]
- 46.Turner JT. Zooplankton fecal pellets, marine snow and sinking phytoplankton blooms. Aquat Microb Ecol. 2002;27:57–102. [Google Scholar]
- 47.Ploug H. Small-scale oxygen fluxes and remineralisation in sinking aggregates. Limnol Oceanogr. 2001;46:1624–1631. [Google Scholar]
- 48.Alldredge AL, Cohen Y. Can microscale chemical patches persist in the sea? Microelectrode study of marine snow, fecal pellets. Science. 1987;235:689–691. doi: 10.1126/science.235.4789.689. [DOI] [PubMed] [Google Scholar]
- 49.Shanks AL, Reeder ML. Reducing microzones and sulfide production in marine snow. Mar Ecol Prog Ser. 1993;96:43–47. [Google Scholar]
- 50.Grossart HP, Dziallas C, Leunert F, Tang KW. Bacteria dispersal by hitchhiking on zooplankton. Proc Natl Acad Sci USA. 2010;107:11959–11964. doi: 10.1073/pnas.1000668107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bonte D, et al. Costs of dispersal. Biol Rev Camb Philos Soc. 2011 doi: 10.1111/j.1469-185X.2011.00201.x. in press. [DOI] [PubMed] [Google Scholar]
- 52.Levin SA, Muller-Landau HC, Nathan R, Chave J. The ecology and evolution of seed dispersal: A theoretical perspective. Annu Rev Ecol Evol Syst. 2003;34:575–604. [Google Scholar]
- 53.Lane DJ. In: Nucleic Acid Techniques in Bacterial Systematics. Stackebrandt E, Goodfellow M, editors. Chichester, UK: John Wiley & Sons, Inc.; 1991. pp. 115–175. [Google Scholar]
- 54.Silhavy TJ, Berman ML, Enquist LW. Experiments with Gene Fusions. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; 1984. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.