Table 2.
Library prototype utilizing dihydroisothiazole 1,1-dioxide 2.
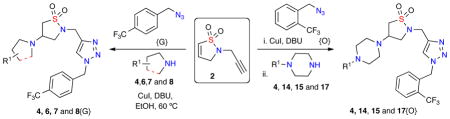 | |||||||
|---|---|---|---|---|---|---|---|
| entrya | yield | final purityc | Mass | entryb | yield | final purityc | mass |
| 4{G} | 52 % | 99 % | 89.3 mg | 4{O} | 43 % | 100 % | 22.9 mg |
| 6{G} | 45 % | 99 % | 75.4 mg | 14{O} | 41 % | 98 % | 22.3 mg |
| 7{G} | 42 % | 92 % | 56.2 mg | 15{O} | 40 % | 97 % | 19.4 mg |
| 8{G} | 47 % | 99 % | 63.9 mg | 16{O} | 43 % | 98 % | 22.3 mg |
Reaction conditions: Dihydroisothiazole 1,1-dioxide 2 (50 mg, 1 equiv.), azide (2 equiv.), amine (1.2 equiv.), CuI (30 mol%), DBU (10 mol%), dry EtOH (0.5 M), 60 °C, 12 hrs.
Reaction conditions: Dihydroisothiazole 1,1-dioxide 2 (20 mg, 1 equiv.), azide (2 equiv.), amine (1.2 equiv.), CuI (30 mol%), DBU (10 mol%), dry EtOH (0.5 M), 60 °C, 12 hrs.
Purified by an automated preparative reverse phase HPLC (detected by mass spectroscopy). Purity was determined by HPLC with peak area (UV) at 214 nm and % rounded up to nearest 1%.
