Fig. 4.
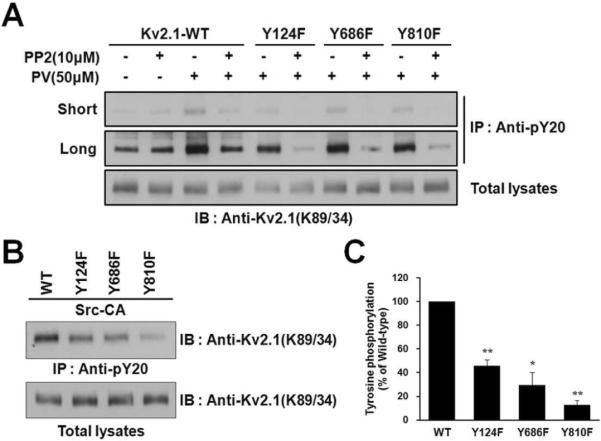
The Y686 and Y810 residues of the Kv2.1 channel are phosphorylated by Src. (A) PV phosphorylates the Y124, Y686, and Y810 residues of Kv2.1 via Src kinase. HEK293 cells were transfected with Kv2.1-WT or mutants (Y124F, Y686F, and Y810F). Cells were pretreated with or without Src kinase inhibitor PP2 before PV treatments. Cells were lysed and immunoprecipitated with anti-pY20 mAb. The immunoprecipitation products were separated by 7.5% SDS-PAGE and immunoblotted with anti-Kv2.1 mAb. Short, short exposure; Long, long exposure. (B) Mutating Y124, Y686 and Y810 decreases tyrosine phosphorylation of Kv2.1. HEK293 cells were co-transfected with Kv2.1-WT or mutants (Y124F, Y686F, and Y810F) and Src-CA. Cells were lysed and immunoprecipitated with anti-pY20 mAb. The immunoprecipitation products were separated by 7.5% SDS-PAGE and immunoblotted with anti-Kv2.1 mAb. (C) Kv2.1 tyrosine phosphorylation levels, normalized to Kv2.1 protein expression, are shown. Data are expressed as the mean ± S.E.M. (n=3, * P < 0.05, ** P < 0.01, Student's t test).
