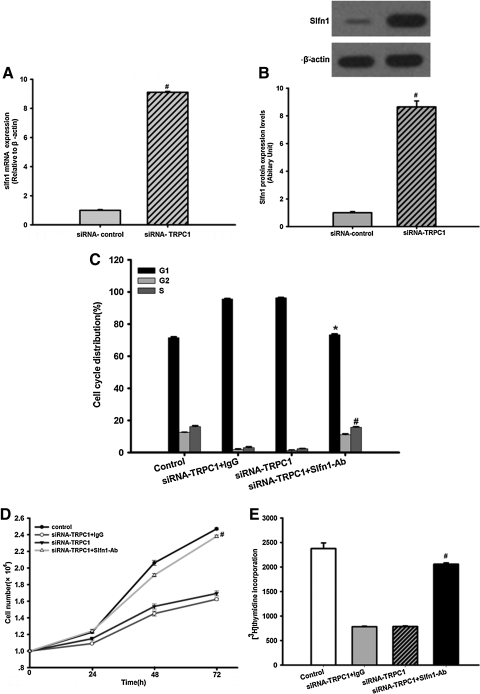
FIG. 4.
The impact of Slfn1 inhibition on the effects of TRPC1 knockdown in EPCs. (A) Slfn1 mRNA levels were determined using real-time RT-PCR. Transduction of EPCs with siRNA-TRPC1 clearly increased Slfn1 mRNA expression at 48 h post-transduction. n=3, #P<0.05 versus siRNA-control. (B) Top: Western blot analysis was used to evaluate Slfn1 protein levels. Equal loading was confirmed by staining of β-actin. Transduction of EPCs with siRNA-TRPC1 significantly increased Slfn1 protein expression after infection for 48 h (n=3). Bottom: Densitometric analysis of Slfn1 protein expression levels, normalized to expression levels of the housekeeping β-actin gene, was performed using the Quantity One program. Data represent the mean±SD from 3 different experiments. n=3, #P<0.05 versus siRNA-control. (C) Impact of Slfn1 inhibition on cell cycle-related effects of TRPC1 knockdown in EPCs. Flow cytometry was used to determine the cell cycle distribution of EPCs transfected with siRNA-TRPC1, in the absence or presence of Slfn1-blocking peptide (Slfn1-Ab, 20 μg/mL) or control goat IgG at 48 h post-transfection. Slfn1-blocking peptide increased the number of cells in S phase and decreased the number of cell in G1 phase, whereas a control antibody did not change the cell cycle distribution (n=3, #P<0.05 vs. siRNA-control S phase. *P<0.05 vs. siRNA-control G1 phase). (D) EPC proliferation was clearly inhibited by siRNA-TRPC1, whereas Slfn1-blocking peptide partially reversed the effects of TRPC1 silencing. Data are presented as the mean±SD (n=3), #P<0.05 versus siRNA-TRPC1. (E) [3H]-thymidine incorporation was used to examine EPC proliferation. Transfection of EPCs with siRNA-TRPC1 significantly decreased the uptake of [3H]-thymidine by EPCs 48 h after infection, whereas Slfn1-blocking peptide partly restored the effects of TRPC1 silencing. Data are presented as the mean±SEM from 3 different experiments, n=9, #P<0.05 versus siRNA-TRPC1. Slfn1, Schlafen 1.