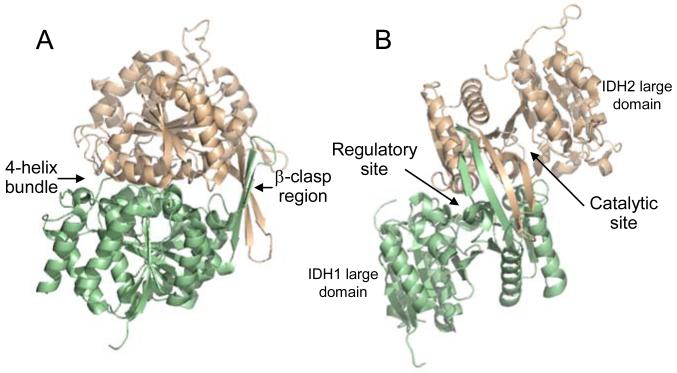
Fig. 1.
The yeast IDH heterodimer. The regulator y IDH1 subunit is shown in light green and the catalytic IDH2 subunit is shown in light gold. (A) Shown are major sites of interaction between the subunits, which are related by a pseudo two-fold axis. Two anti-parallel β-strands of each subunit align with the same strands of the other subunit forming a β-clasp region. A four-helix bundle is formed by two analogous alpha helices from each subunit. (B) A 90o rotation of the structure is used to indicate the positions of large domains (which contribute little to subunit interactions) and of active sites in each subunit. The heterodimer structure is from the ligand-free structure of IDH (PDB ID: 3BLX), and the orientation of IDH1 and IDH2 subunits in the heterodimer in (A) is maintained in upper left portions of structures shown in Fig. 2.