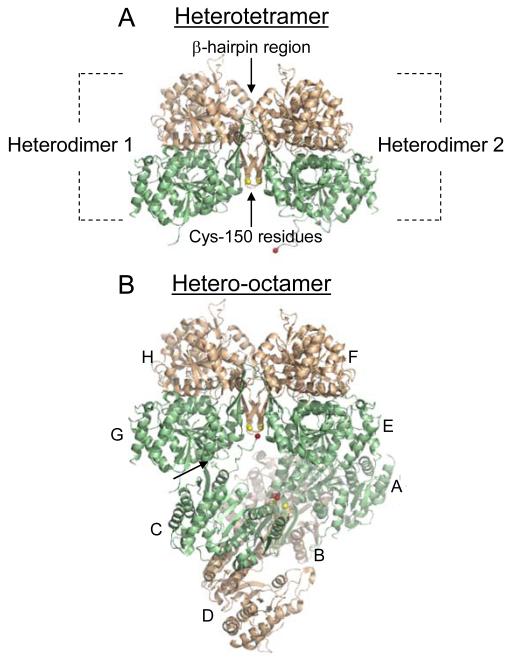
Fig. 2.
The IDH heterotetramer and hetero-octamer. Regulatory IDH1 subunits are shown in light green and catalytic IDH2 subunits are shown in light gold. (A) The major interaction between heterodimers in the heterotetramer is a β-hairpin structure formed by the β-clasp regions from each heterodimer. Cys-150 residues from IDH2 subunits located on one side of the β-hairpin structure are shown in yellow. (B) Heterodimers (A/B, C/D, E/F, and G/H) that form the octameric enzyme are indicated. In the octamer, one heterotetramer (A/B and C/D) is twisted relative to the other heterotetramer (E/F and G/H). The heterotetramers interact primarily by a protrusion of the amino terminus from an IDH1 subunit in one tetramer (e.g. C) into a pocket of an IDH1 subunit in the other tetramer (e.g. G), as indicated by an arrow, positioning the amino terminus of the C subunit (shown in red) in the vicinity of the IDH2 Cys-150 residues in the other tetramer. The heterotetramer and hetero-octamer structures are from the citrate-bound structure of IDH (PDB ID: 3BLV).