Table 2.
Leishmania CRK3–CYC6 and HsCDK2–CYCA activity (compounds 14–39) from initial SAR development at the R1 position of triazolopyridine 19
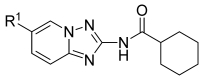 | |||
|---|---|---|---|
| Compd | R1 | CRK3–CYC6 IC50 [μm][a] | HsCDK2–CYCA IC50 [μm][b] |
| 14 | 4-hydroxy-3-methoxyphenyl | 0.23 | 0.36 |
| 21 | H | >100 | >100 |
| 22 | phenyl | 0.27 | >100 |
| 23 | 4-hydroxyphenyl | 0.12 | 0.86 |
| 24 | 3-methoxyphenyl | 0.36 | >100 |
| 25 | 3-methylphenyl | 0.06 | >100 |
| 26 | 3-chlorophenyl | 0.35 | >100 |
| 27 | 1-napthyl | 0.09 | >100 |
| 28 | 4-indole | 0.07 | >100 |
| 29 | 4-N-methylindole | 0.04 | >100 |
| 30 | 3-morpholinophenyl | 1.6 | >100 |
| 31 | 2,6-dimethylphenyl | 19.0 | >100 |
| 32 | 2,5-dimethylphenyl | 0.65 | >100 |
| 33 | 3,5-dimethylphenyl | 0.02 | >100 |
| 34 | 4-methoxy-3-methylphenyl | 0.03 | >100 |
Concentration required to inhibit Leishmania CRK3–CYC6 activity by 50 %; data represent the mean of two or more experiments.
Concentration required to inhibit HsCDK2–CYCA activity by 50 %; data represent the mean of two or more experiments.
