Table 3.
Leishmania CRK3–CYC6 and HsCDK2–CYCA activity from initial SAR investigations at the R position of triazolopyridine 19 (Scheme 3) and a benzimidazole core
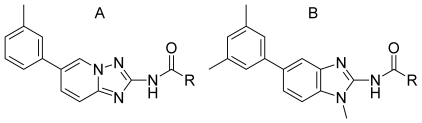 | ||||
|---|---|---|---|---|
| Compd | Core | R | CRK3–CYC6 IC50 [μm][a] | HsCDK2–CYCA IC50 [μm][b] |
| 35 | A | free NH2, no amide | >100 | 37 |
| 36 | A | cyclohexylmethyl, no amide | 3.0 | 64 |
| 37 | A | methyl | 65 | 26.0 |
| 38 | A | 2-tetrahydrofuran | 12 | >100 |
| 39 | A | 4-tetrahydropyran | 10 | >100 |
| 40 | A | 3-piperidine | 12 | >100 |
| 41 | A | phenyl | 1.1 | >100 |
| 42 | A | cyclobutyl | 3.3 | >100 |
| 43 | A | cyclopentyl | 1.1 | >100 |
| 44 | A | cyclopentylmethyl | 0.04 | >100 |
| 45 | A | cyclohexylmethyl | 0.01 | >100 |
| 46 | A | cyclopentylethyl | <0.005 | >100 |
| 47 | A | phenethyl | 0.03 | >100 |
| 48 | B | cycloheptyl | <0.005 | >100 |
Concentration required to inhibit Leishmania CRK3–CYC6 activity by 50 %; data represent the mean of two or more experiments.
Concentration required to inhibit HsCDK2–CYCA activity by 50 %; data represent the mean of two or more experiments.
