Abstract
Background
Activating enhancer-binding protein-2β (AP2β) is a transcription factor involved in apoptosis. The purpose of the current study was to assess the cellular location and level of AP2β in Non-Small Cell Lung Cancer (NSCLC) and normal lung tissue and investigate whether the level and localization of AP2β expression is predictive of overall survival in patients with stage I NSCLC.
Methods
We performed immunohistochemical analysis of tissue microarrays (TMAs) prepared from stage I NSCLC specimens with adjacent normal lung tissue from two independent sets of patients who underwent lung resection with curative intent at our institution. AP2β intensity was assessed in TMAs, and AP2β staining patterns were classified as either diffuseor nucleolar in the TMAs. AP2β intensity and localization were analyzed for correlation with patients' survival.
Results
Immunohistochemical analysis of TMAs showed that the intensity of AP2β immunohistochemical staining did not correlate with overall survival. When location of AP2β was analyzed in TMAs, all of the normal lung tissue had diffuse pattern of AP2β. In the first set of NSCLC, patients with nucleolar pattern had a significantly lower 5-year survival rate than patients with diffuse pattern (67% vs. 100%; P = 0.004); this finding was confirmed in the second set (64% vs. 91%; P = 0.02). Multivariate analysis revealed that nucleolar pattern was an independent predictor of poor overall survival in both sets.
Conclusions
The AP2β which is located in the nucleoplasm in normal lung tissue is found in either nucleoplasm or nucleoli in NSCLC. The patients with AP2β in the nucleoli had poor survival compared to patients with AP2β in the cytoplasm.
Keywords: Lung cancer biology, survival analysis
Introduction
The overall 5-year survival rate among patients with stage I non-small cell lung cancer (NSCLC) who undergo resection with curative ranges from 55% to 72%.1 This poor survival for the early stage cancer suggest that lung cancer is very aggressive and identifying the biomarkers that underlie the aggressiveness of NSCLC could be used to identify patients at high risk of recurrence and death and subsequently guide adjuvant therapy decisions to improve overall survival.
One known prognostic factor in patients with stage I NSCLC is human telomerase reverse transcriptase (hTERT), an important component of human telomerase, the enzyme that lengthens chromosome ends to prevent apoptosis in cancer cells. When hTERT is not expressed in a cancer cell, the cell will reach its Hayflick limit and eventually undergo apoptosis; when hTERT is overexpressed, telomere length is maintained, and the cancer cell avoids apoptosis. hTERT may localize in the nucleoli of cancer cells, or it may localize in the nucleoplasm, where its telomere-lengthening activity occurs.2, 3 In NSCLC patients, hTERT expression has been associated with lower rates of overall and disease-free survival.4 The hTERT expression, as well as the expression of several other oncogenes,5 is regulated by the transcription factor activating enhancer-binding protein-2β (AP2β).6 AP2β is involved in development, differentiation, and carcinogenesis in mammals.7–10 Gene knockout experiments with AP2β have shown that AP2β-deficient mice die shortly after birth due to collecting duct and distal tubular epithelia defects caused by massive renal epithelial cell apoptosis.11 These findings suggests that AP2β is important in preventing cell death and that AP2β expression in NSCLC may lead to tumors that are resistant to signals for apoptosis.
In the current study, we investigated whether hTERT and AP2β co-localize in the nuclei of NSCLC cells and whether the level and localization of AP2β expression is predictive of overall survival in patients with stage I NSCLC.
Material and Methods
Assessing AP2β Expression in Lung Cancer Cells
Western blot analysis
Immortalized normal human bronchial epithelial cells (HBECs) and H1299 and A549 NSCLC cells were grown to 80% confluence under cell culture conditions as described previously.12 Cell lysate was prepared in 1× Lamini/urea buffer containing 62.5 mM Tris-HCl (pH, 6.8), 2% SDS (w/v), 5% glycerol, 6 M of urea, and complete protease inhibitors (Roche Applied Science, Indianapolis, IN). Protein concentrations were determined using the BCA Protein Assay Kit (Thermo Scientific, Waltham, MA). Next, 50 μg of crude protein extracts were loaded onto a 10% SDS-PAGE gel and run for approximately 150 min at 130 V. The separated proteins were then transferred to a nitrocellulose membrane and run for 1 h at 100 V. The membrane was incubated with blocking buffer containing 5% milk in PBS for 1 h at room temperature. After blocking, the membrane was incubated simultaneously with AP2β and β-actin antibodies at dilutions of 1:2500 and 1:10000, respectively, in Tris-buffered saline with 0.1% Tween-20 and 5% milk. Odyssey secondary antibodies (goat anti-rabbit IRDye 800 and goat anti-mouse IRDye 680; Li-COR Biosciences, Lincoln, NE) were added according to the manufacturer's instructions. Blots were imaged using an Odyssey Infrared Imaging System (Li-COR Biosciences).
Immunohistochemical analysis
For immunohistochemistry of the immortalized HBECs and H1299 and A549 NSCLC cells, 5-μm-thick, paraffin-fixed cell-block sections were deparaffinized in xylene and rehydrated with graded alcohols. Antigen retrieval was carried out using BORG decloaker buffer (Biocare Medical LLC, Concord, CA). Endogenous peroxidase activity was quenched by immersion in 3% hydrogen peroxide (Sigma-Aldrich, St. Louis, MO) or methanol at room temperature. Nonspecific primary antibody binding was blocked by incubating the sections with 10% normal goat serum. The sections were then incubated with a rabbit polyclonal antibody against AP2β (1:150 dilution; Santa Cruz Biotechnology, Santa Cruz, CA) overnight at 4°C. Next, the slides were incubated with secondary anti-rabbit IgG-biotin antibody (1:200 dilution; Vectastain Elite ABC Kit; Vector laboratories, CA) for 1 h at room temperature. Visualization was performed using chromogen 3,3′-diaminobenzidine (Dako, Carpinteria, CA). The slides were counterstained with hematoxylin and coverslipped with PerMount. Cell block sections that were incubated without the primary antibody were used as negative controls.
Patients and Tissue Samples
This retrospective study was approved by The University of Texas MD Anderson Cancer Center Institutional Review Board. We looked at two independent sets of patients with stage I lung cancer since the median follow-up in our first set of patient was very short. We felt that analysis in a second group of patients with longer median follow-up would strengthen our conclusions.
Patient sets
Two independent sets that were created by two different departments at the M.D. Anderson Cancer Center were analyzed for the study. The first set of tissue microarray was created from NSCLC specimens (stage I squamous cell carcinoma and stage I adenocarcinoma) from 126 patients who underwent lung resection with curative intent at MD Anderson Cancer Center between 2002 and 2008. Normal lung tissue specimens adjacent to tumor were available for 86 of these patients. The second set of tissue microarray was comprised of NSCLC specimens (stage I squamous cell carcinoma and stage I adenocarcinoma) from 115 patients who underwent lung cancer resection with curative intent at MD Anderson Cancer Center between 1994 and 2004. Although there was two-year overlap between the sets, there were no patients who were in both sets. NSCLC stage was determined according to the 7th edition of the American Joint Committee on Cancer lung cancer staging13. Patients' clinicopathological data were obtained from a clinical database derived from patient charts and electronic medical record.
Immunohistochemical analysis
Immunohistochemical analysis was performed to assess the intensity and location of AP2β and location of hTERT in the NSCLC and location of AP2β in adjacent normal lung tissue in first patient set and location of AP2β in second patient set. Tissue microarrays were incubated with 0.3% hydrogen peroxide for 5 min to inactivate the endogenous peroxidase and then in 5% normal goat serum for 1 h at room temperature to eliminate non-specific protein binding. The microarrays were then incubated with a rabbit polyclonal antibody against AP2β (1:150 dilution; Santa Cruz Biotechnology) and a mouse monoclonal antibody against hTERT (1:40 dilution; Novus Biological, Littleton, CO) overnight at 4°C. Next, the microarrays were washed with phosphate-buffered saline (PBS) for 5 min and then incubated with a biotinylated second antibody at room temperature for 1 h. The microarrays were then washed with PBS for 5 min, incubated with rabbit Extra Avidin peroxidase reagent (Sigma-Aldrich) for 30 min, and washed again with PBS for 10 min. Color was developed using 0.05% 3,3 diaminobenzine hydrochloride (Sigma-Aldrich) and 0.0006% hydrogen peroxide in PBS. The microarrays were then counterstained with Mayer's hematoxylin, dehydrated, cleared, and mounted. Microarrays of NSCLC and normal lung tissue incubated with PBS containing 1% bovine serum albumin without the primary antibodies were used as negative controls.
Two investigators (RK and YC) independently assessed the intensity and pattern of AP2β staining and the pattern of hTERT staining in the NSCLC and normal lung tissue specimens. AP2β staining intensity scores were calculated by multiplying the percentage of cells stained the nucleoplasm and/or nucleoli by the intensity score (1, 2, 3, or 4) of the stain. Staining of the nucleoplasm without nucleoli staining was labeled as diffuse, and staining of several small nucleoli or a single large macro-nucleoli with or without nucleoplasm staining was labeled as nucleolar.
Statistical analysis
Statistical analyses were performed on the two independently assessed datasets of AP2β staining intensity and pattern and hTERT staining pattern. Identical statistical methods were applied for both sets. AP2β staining patterns, staining intensity score, and histology were correlated with patients' clinicopathological data. AP2β and hTERT staining patterns were analyzed for correlation using the Fisher's Exact Initial. The Wilcoxon rank-sum initial was used to analyze the distribution of age, tumor size, and clinicopathological features according to AP2β staining pattern and intensity. The Kruskal-Wallis rank-sum test was used to assess the distribution of AP2β intensity scoring by tumor differentiation. Fisher's exact test was used to assess relationships between AP2β staining patterns and gender, histology, stage, differentiation, and smoking status. Pearson correlation was calculated to assess the relationship between AP2β intensity and age and tumor size. The Kaplan-Meier method was used to estimate patients' overall survival rate, and the log-rank test was used to compare the survival distributions in patients with diffuse AP2β staining and patients with nucleolar AP2β staining. Univariate analysis was performed on age, gender, smoking history, histology, tumor differentiation, size of tumor, AP2β intensity and AP2β pattern for survival, and multivariate analysis was performed using standard criteria. Because no deaths occurred among the patients in the initial set whose disease had diffuse AP2β staining, Firth's penalized maximum likelihood bias reduction method for the Cox proportional hazards regression model was used for multivariate analysis of overall survival in these patients. P values < 0.05 were considered statistically significant. Hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated for the multivariate analysis for overall survival. All statistical analyses were performed using SAS version 9.2.0 and SPLUS version 7 (SAS Institute, Inc., Cary, NC).
Results
AP2β Expression in Lung Cancer Cells
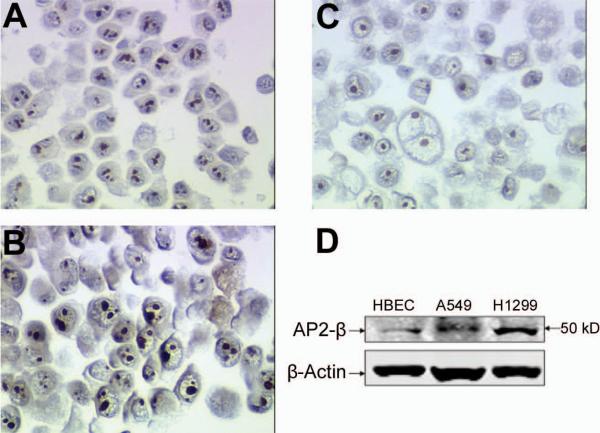
Immunohistochemistry and Western blot revealed that AP2β levels were higher in the H1299 and A549 NSCLC cells than in the HBECs (Fig. 1).
FIGURE 1.
Both A549 (a) and H1299 (b) lung cancer cell have more AP2β staining compared to control immortalized HBEC (c) cells. Western-blot analysis showed that AP2β protein expression is upregulated in the A549 and H1299 lung cancer cells compared to control immortalized HBEC cells (d).
First Patient Set
The first patient set included 59 men and 67 women. The mean age was 67 years. Fifteen patients (12%) were never-smokers. Most tumors were adenocarcinomas (69%), and most tumors were either moderately (47%) or poorly (39%) differentiated. The mean follow-up time was 23 months.
AP2β intensity scores did not correlate with patients' gender, age, or smoking status or tumor size, histology, or differentiation (Table 1). The log-rank test did not reveal an association between AP2β intensity and 5-year overall survival rate (HR, 1.001; 95% CI, 0.99-1.01). Thirty-five patients had a diffuse AP2β pattern, and 91 patients had a nucleolar AP2β pattern (Fig 2). We found no significant differences in patient age, tumor size, or tumor histology between patients with diffuse AP2β staining and patients with nucleolar AP2β staining; however, we did find that significantly more women than men had a diffuse AP2β staining pattern (P = 0.046, Table 2). Moreover, significantly more patients with poorly differentiated adenocarcinomas had a nucleolar AP2β pattern than a diffuse AP2β pattern (P = 0.011), and significantly more never-smokers had a diffuse AP2β pattern than a nucleolar AP2β pattern (P = 0.029). All 86 normal lung tissue specimens had diffuse AP2β staining (Fig. 2).
TABLE 1.
Relationship Between Activating Enhancer-Binding Protein-2β (AP2β) Staining Intensity in Stage I Non–Small Cell Lung Cancer and Patients' Clinicopathological Features in the First Patient Set
| AP2β Staining Intensity |
||||
|---|---|---|---|---|
| Characteristic | No. of Patients | Mean ± SD | Median (range) | p |
| Sex | ||||
| Male | 59 | 263 ± 67 | 270 (88–380) | 0.52 |
| Female | 67 | 255 ± 63 | 270 (102–380) | |
| Tumor histology | ||||
| Adenocarcinoma | 87 | 258 ± 65 | 275 (101–380) | 0.92 |
| Squamous cell carcinoma | 39 | 262 ± 65 | 270 (88–380) | |
| Smoking status | ||||
| Never | 15 | 258 ± 71 | 285 (140–370) | 0.82 |
| Ever | 111 | 259 ± 64 | 270 (88–380) | |
| Tumor differentiationa | ||||
| Well | 11 | 252 ± 56 | 275 (175–360) | 0.6 |
| Moderate | 59 | 263 ± 69 | 277 (88–380) | |
| Poor | 49 | 253 ± 63 | 261 (102–380) | |
Tumor differentiation data for seven patients were unavailable.
SD, standard deviation; NS, not significant.
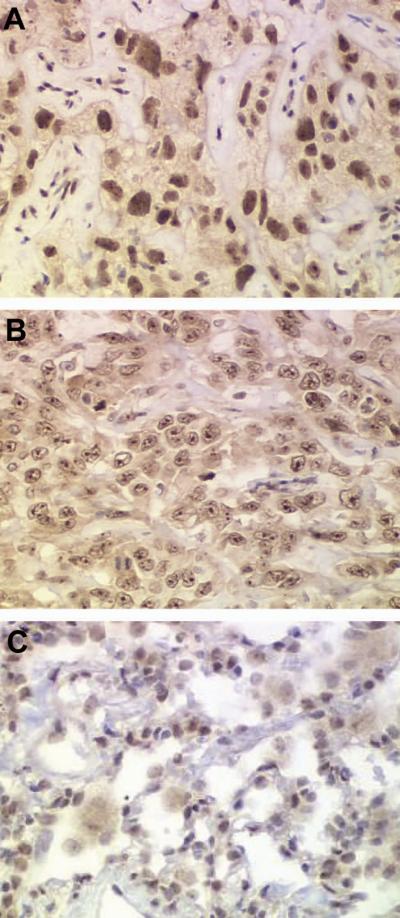
FIGURE 2.
Diffuse nuclear AP2β staining characterized by nucleoplasm staining without nucleoli staining in NSCLC (a) Nucleolar AP2β staining characterized by the staining of several small nucleoli or a single large macronucleoli with or without nucleoplasm staining in NSCLC (b). All tumor-adjacent normal lung tissue specimens had diffuse nuclear AP2β staining (c).
TABLE 2.
Relationship Between Activating Enhancer-Binding Protein-2β (AP2β) Staining Pattern in Stage I Non-Small Cell Lung Cancer and the Clinicopathological Features of the First Patient Seta
| AP2β Staining Pattern |
||||
|---|---|---|---|---|
| Characteristic | Total, n = 126 | Diffuse, n = 35 | Nucleolar, n = 91 | p |
| Mean age ± SD, y | 66 ± 18 | 64 ± 16 | 0.31 | |
| Sex | ||||
| Male | 59 (47) | 11 (31) | 48 (53) | 0.04 |
| Female | 67 (53) | 24 (69) | 43 (47) | |
| Smoking | ||||
| Never | 15 (12) | 8 (23) | 7 (8) | 0.03 |
| Ever | 111 (88) | 27 (77) | 84 (92) | |
| Histology | ||||
| Squamous cell carcinoma | 39 (31) | 8 (23) | 31 (34) | 0.28 |
| Adenocarcinoma | 87 (69) | 27 (77) | 60 (66) | |
| Differentiationb | ||||
| Well | 11 (9) | 7 (20) | 4 (4) | 0.01 |
| Moderate | 59 (47) | 17 (49) | 42 (46) | |
| Poor | 49 (39) | 9 (26) | 40 (44) | |
| Mean tumor size ± SD, cm | 2.7 ± 1 | 3.2 ± 1.7 | 0.24 | |
Data are given as no. of patients (%) unless otherwise indicated.
Tumor differentiation data for seven patients were unavailable.
SD, standard deviation; NS, not significant.
The Kaplan-Meier survival plot revealed that patients with a nucleolar AP2β staining pattern had a significantly lower 5-year survival rate (67%) than patients with a diffuse AP2β staining pattern (100%; P = 0.004; Fig. 3). Multivariate analysis revealed that cellular AP2β location was an independent predictor of 5-year survival (P = 0.004; HR, 13.9; 95% CI, 1.9–1756). We determined the cellular location of hTERT in 96 patients. Twenty-seven patients had a diffuse pattern of hTERT staining, and 69 patients had a nucleolar pattern of hTERT staining. hTERT localization and AP2β localization were correlated (P < 0.0001).
FIGURE 3.
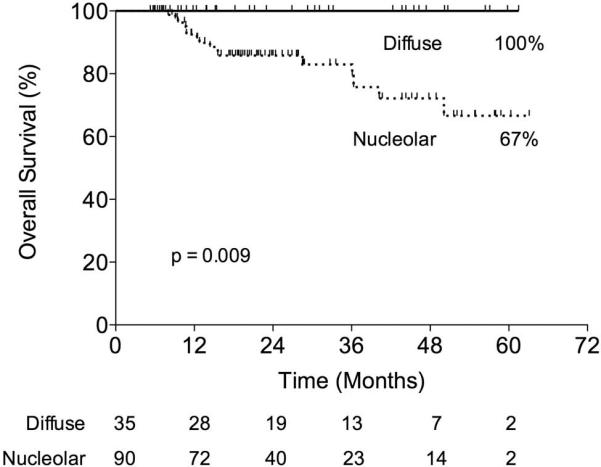
In the initial set, patients who had NSCLC with a nucleolar AP2β pattern had a significantly lower 5-year survival rate than patients who had NSCLC with a diffuse AP2β pattern (67% vs. 100%; p = 0.009).
Second Patient Set
The second set included 57 men and 58 women. The mean age was 68 years, and the majority of tumors were adenocarcinomas (72%). Twenty patients (17%) were never-smokers, 50 patients (43%) had a poorly differentiated tumor, and 93 patients (81%) underwent lobectomy for NSCLC. The mean follow-up time was 83 months.
Thirty-five patients had a diffuse AP2β staining pattern, and 80 patients had a nucleolar AP2β staining pattern. We found no significant differences in age, tumor size, tumor histology, smoking status, tumor differentiation, race, or surgery type between patients with a diffuse AP2β pattern and patients with a nucleolar AP2β pattern (Table 3). However, significantly more women than men had a diffuse AP2β pattern (P = 0.04). The Kaplan-Meier survival plot revealed that patients with a diffuse AP2β pattern had a significantly higher 5-year survival rate than did patients with a nucleolar AP2β pattern (91% vs. 64%; P = 0.0211; Fig. 4). Univariate and multivariate analyses revealed that AP2β staining pattern (P = 0.037; HR, 2.18; 95% CI, 1.05–1.47) and patient age (P = 0.001; HR, 1.07; 95% CI, 1.03–1.11) were independent predictors of 5-year survival.
TABLE 3.
Relationship Between Activating Enhancer-Binding Protein-2β (AP2β) Staining Pattern in Stage I Non-Small Cell Lung Cancer and the Clinicopathological Features of the Second Patient Seta
| AP2β Staining Pattern |
||||
|---|---|---|---|---|
| Characteristic | Total, n = 115 | Diffuse, n = 35 | Nucleolar, n = 80 | p value |
| Sex | ||||
| Male | 57 (50) | 12 (34) | 45 (49) | 0.04 |
| Female | 58 (50) | 23 (66) | 35 (38) | |
| Mean age ± SD, y | 66 ± 10 | 68 ± 11 | 0.32 | |
| Smoking | ||||
| Never | 20 (17) | 8 (23) | 12 (15) | 0.57 |
| Former | 55 (48) | 15 (43) | 40 (50) | |
| Current | 40 (35) | 12 (34) | 28 (35) | |
| Histology | 1 | |||
| Squamous cell carcinoma | 32 (28) | 10 (29) | 22 (24) | |
| Adenocarcinoma | 83 (72) | 25 (71) | 58 (64) | |
| Differentiation | ||||
| Well | 32 (28) | 10 (29) | 22 (24) | 0.3 |
| Moderate | 33 (29) | 11 (31) | 22 (24) | |
| Poor | 50 (43) | 14 (40) | 36 (40) | |
| Mean tumor size ± SD, cm | 3.1 ± 1 | 2.8 ± 0.9 | 0.07 | |
Data are given as no. of patients (%) unless otherwise indicated.
SD, standard deviation; NS, not significant.
FIGURE 4.
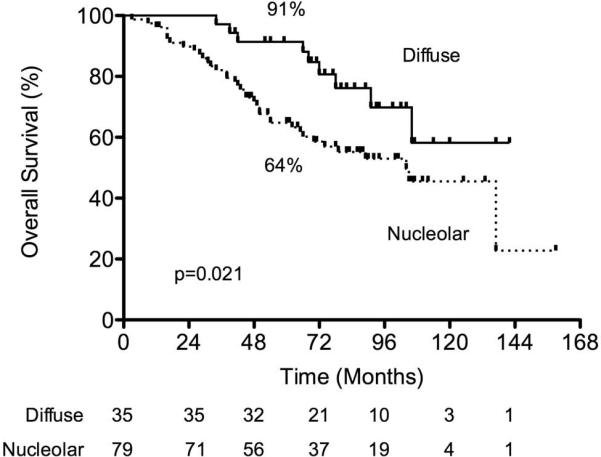
In the validation set, patients who had NSCLC with a nucleolar AP2β pattern had a significantly lower 5-year survival rate than patients who had NSCLC with a diffuse AP2β pattern (64% vs. 91%; p = 0.0211).
Comment
The AP2β is a transcription factor that plays an important role in regulating apoptosis and control cell division14 however there is limited information about its role in lung cancer. In our study, the AP2β which is located in the nucleoplasm in normal lung tissue was found in either nucleoplasm or nucleoli in NSCLC and the patients with AP2β in the nucleoli had poor survival. Most prognostic markers for NSCLC are identified by assessing the level of RNA or a specific protein in the tumor and then determining whether that level correlates with survival. Immunohistochemistry studies have shown that Ki-67, p53, and Bcl-2 are important in NSCLC and may provide some prognostic information.15 We took a similar approach and investigated the level of AP2β and its relation to survival. Since presence of hTERT has been associated with survival in NSCLC patients,4 we expected that the hTERT promoter AP2β would also be associated with survival. Surprisingly, we found no relationship between the level of AP2β and survival. Although AP2β was expressed in normal lung tissue as well as in NSCLC and that higher AP2β levels were expressed in the NSCLC cell lines than in the normal cells, AP2β level was not a predictor of survival. The difference may be due to how hTERT levels were determined in previous study.4 Wang et al. found the hTERT level by performing in situ hybridization for hTERT mRNA expression. They looked at the presence of the hTERT mRNA in the cytoplasm of NSCLC cells and found that only 33% of the samples expressed hTERT. In the current study, we found that hTERT was expressed in 76% of the samples studied, which is much closer to 86% of samples identified in other studies.16 Thus, in situ hybridization method that was used in Wang et al.4 might not reflect the protein levels and protein levels may not provide prognostic information.
However, in both sets of patients, the cellular location of AP2β was a prognostic marker for survival. In normal lung tissue cells, AP2β was diffusely expressed in the nucleoplasm; in contrast, in the NSCLC cells, AP2β was either diffusely expressed in the nucleoplasm or expressed in the nucleoli. In both patient sets, AP2β localization in the nucleoli, which plays an important role in ribosome biogenesis, was found to be an indicator of poor prognosis. These findings suggest that NSCLC tumors are more resistant to cell death when the AP2β is localized in the nucleoli. Other studies have shown that apoptosis-related proteins are present in the nucleoli after the induction of cell death.17 Since AP2β knockout mice die from massive apoptosis,11 the presence of AP2β in the nucleoli may indicate that this protein is present to prevent cell death and allowing the tumor to grow without any controls.
The current study was not without its limitations. One potential limitation was that the current study was retrospective; thus, some data were unavailable for analysis such as type of resection, morbidity of the operation, type of recurrence and cause of death. A larger issue stemming from the retrospective nature of the study was the fact that the patient groups were not balanced. In the first set of patients, more women, never-smokers, and patients with well differentiated tumors were in the diffuse staining group than in the nucleolar staining group, whereas in the second set of patients, more women were in the diffuse staining group than in the nucleolar staining group. To account for the unbalanced group, we used univariate and multivariate analyses, which accounts for these factors, to analyze the relationship between different factors and survival and we found that AP2β nucleolar localization was independent predictor of poor survival. Moreover, in the second patient set, age was also an independent predictor of survival which suggests that patients who undergo lung cancer resection are at a higher risk of dying from other causes such as cardiovascular disease. Thus, later deaths among patients in the validation set may have been due to factors that were independent of tumor aggressiveness but associated with advanced age and its comorbidities. Moreover, our use of two independent groups of patients strengthened the study, with findings in the first set also seen in the second set of patients.
In conclusion, the current study's findings show that AP2β co-localizes with hTERT in either the nucleoplasm or nucleoli of NSCLC cells. Moreover, the AP2β nucleolar localization was associated with very poor survival. The current standard of care for patients with stage I NSCLC is surgical resection. If the AP2β nucleolar pattern is indeed an indicator of poor prognosis, these patients may benefit from adjuvant therapy to improve their survival. Additional studies are needed to confirm our findings, identify the exact mechanism of nucleolar localization of the AP2β and aggressiveness of tumor and determine whether adjuvant therapy prolongs survival in patients predicted to have poor prognosis.
Acknowledgements
This work was supported in part by the National Institutes of Health through MD Anderson's Cancer Center Support Grant CA-016672 – Lung Program, Biostatistics CCSG Shared Resource; Specialized Program of Research Excellence (SPORE) Grant CA-070907 (J. Roth); and Department of Defense Vanguard Investigations of Therapeutic Approaches to Lung Cancer (VITAL) Grant W81XWH-04-1-0142 (I. Wistuba). We thank Joe Munch in MD Anderson's Department of Scientific Publications for editing the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nesbitt JC, Putnam JB, Jr., Walsh GL, et al. Survival in early-stage non-small cell lung cancer. Ann Thorac Surg. 1995;60(2):466–72. doi: 10.1016/0003-4975(95)00169-l. [DOI] [PubMed] [Google Scholar]
- 2.Yan P, Benhattar J, Seelentag W, et al. Immunohistochemical localization of hTERT protein in human tissues. Histochem Cell Biol. 2004;121(5):391–7. doi: 10.1007/s00418-004-0645-5. [DOI] [PubMed] [Google Scholar]
- 3.Etheridge KT, Banik SS, Armbruster BN, et al. The nucleolar localization domain of the catalytic subunit of human telomerase. J Biol Chem. 2002;277(27):24764–70. doi: 10.1074/jbc.M201227200. [DOI] [PubMed] [Google Scholar]
- 4.Wang L, Soria JC, Kemp BL, et al. hTERT expression is a prognostic factor of survival in patients with stage I non-small cell lung cancer. Clin Cancer Res. 2002;8(9):2883–9. [PubMed] [Google Scholar]
- 5.Eckert D, Buhl S, Weber S, et al. The AP-2 family of transcription factors. Genome Biol. 2005;6(13):246. doi: 10.1186/gb-2005-6-13-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ma H, Urquidi V, Wong J, et al. Telomerase reverse transcriptase promoter regulation during myogenic differentiation of human RD rhabdomyosarcoma cells. Mol Cancer Res. 2003;1(10):739–46. [PubMed] [Google Scholar]
- 7.Ding X, Fan C, Zhou J, et al. GAS41 interacts with transcription factor AP-2beta and stimulates AP-2beta-mediated transactivation. Nucleic Acids Res. 2006;34(9):2570–8. doi: 10.1093/nar/gkl319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Williams T, Admon A, Luscher B, et al. Cloning and expression of AP-2, a cell-type-specific transcription factor that activates inducible enhancer elements. Genes Dev. 1988;2(12A):1557–69. doi: 10.1101/gad.2.12a.1557. [DOI] [PubMed] [Google Scholar]
- 9.Moser M, Imhof A, Pscherer A, et al. Cloning and characterization of a second AP-2 transcription factor: AP-2 beta. Development. 1995;121(9):2779–88. doi: 10.1242/dev.121.9.2779. [DOI] [PubMed] [Google Scholar]
- 10.Bosher JM, Totty NF, Hsuan JJ, et al. A family of AP-2 proteins regulates c-erbB-2 expression in mammary carcinoma. Oncogene. 1996;13(8):1701–7. [PubMed] [Google Scholar]
- 11.Moser M, Pscherer A, Roth C, et al. Enhanced apoptotic cell death of renal epithelial cells in mice lacking transcription factor AP-2beta. Genes Dev. 1997;11(15):1938–48. doi: 10.1101/gad.11.15.1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nishizaki M, Sasaki J, Fang B, et al. Synergistic tumor suppression by coexpression of FHIT and p53 coincides with FHIT-mediated MDM2 inactivation and p53 stabilization in human non-small cell lung cancer cells. Cancer Res. 2004;64(16):5745–52. doi: 10.1158/0008-5472.CAN-04-0195. [DOI] [PubMed] [Google Scholar]
- 13.Goldstraw P, Crowley J, Chansky K, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol. 2007;2(8):706–14. doi: 10.1097/JTO.0b013e31812f3c1a. [DOI] [PubMed] [Google Scholar]
- 14.Hilger-Eversheim K, Moser M, Schorle H, et al. Regulatory roles of AP-2 transcription factors in vertebrate development, apoptosis and cell-cycle control. Gene. 2000;260(1–2):1–12. doi: 10.1016/s0378-1119(00)00454-6. [DOI] [PubMed] [Google Scholar]
- 15.Zhu CQ, Shih W, Ling CH, et al. Immunohistochemical markers of prognosis in non-small cell lung cancer: a review and proposal for a multiphase approach to marker evaluation. J Clin Pathol. 2006;59(8):790–800. doi: 10.1136/jcp.2005.031351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Albanell J, Lonardo F, Rusch V, et al. High telomerase activity in primary lung cancers: association with increased cell proliferation rates and advanced pathologic stage. J Natl Cancer Inst. 1997;89(21):1609–15. doi: 10.1093/jnci/89.21.1609. [DOI] [PubMed] [Google Scholar]
- 17.Horky M, Kotala V, Anton M, et al. Nucleolus and apoptosis. Ann N Y Acad Sci. 2002;973:258–64. doi: 10.1111/j.1749-6632.2002.tb04645.x. [DOI] [PubMed] [Google Scholar]