Abstract
Objective
Ganglioside-induced differentiation associated-protein 1 (GDAP1) mutations are commonly associated with autosomal recessive Charcot-Marie-Tooth (ARCMT) neuropathy; however, in rare instances, they also lead to autosomal dominant Charcot-Marie-Tooth (ADCMT). We aimed to investigate the frequency of disease-causing heterozygous GDAP1 mutations in ADCMT and their associated phenotype.
Methods
We performed mutation analysis in a large cohort of ADCMT patients by means of bidirectional sequencing of coding regions and exon-intron boundaries of GDAP1. Intragenic GDAP1 deletions were excluded using an allele quantification assay. We confirmed the pathogenic character of one sequence variant by in vitro experiments assaying mitochondrial morphology and function.
Results
In 8 Charcot-Marie-Tooth disease (CMT) families we identified 4 pathogenic heterozygous GDAP1 mutations, 3 of which are novel. Three of the mutations displayed reduced disease penetrance. Disease onset in the affected individuals was variable, ranging from early childhood to adulthood. Disease progression was slow in most patients and overall severity milder than typically seen in autosomal recessive GDAP1 mutations. Electrophysiologic changes are heterogeneous but compatible with axonal neuropathy in the majority of patients.
Conclusions
With this study, we broaden the phenotypic and genetic spectrum of autosomal dominant GDAP1-associated neuropathies. We show that patients with dominant GDAP1 mutations may display clear axonal CMT, but may also have only minimal clinical and electrophysiologic abnormalities. We demonstrate that cell-based functional assays can be reliably used to test the pathogenicity of unknown variants. We discuss the implications of phenotypic variability and the reduced penetrance of autosomal dominant GDAP1 mutations for CMT diagnostic testing and counseling.
Charcot-Marie-Tooth (CMT) disease forms a clinically and genetically heterogeneous group of inherited peripheral neuropathies affecting 1 in 2,500 individuals.1 Mutations in ganglioside-induced differentiation-associated protein 1 (GDAP1) cause autosomal recessive demyelinating,2 axonal,3 and intermediate forms of CMT.4,5 Autosomal recessive GDAP1 patients develop distal muscle weakness and wasting, typically with early childhood onset and a severe disease course. Proximal muscles become affected later, often leading to a wheelchair-dependency in the second or third decade of life. In the majority of patients, sensory impairment is evident on physical examination.6,7 Development of unilateral or bilateral vocal fold paresis (VFP) in the later stages of disease may be indicative of phenotype severity.8 Clinical heterogeneity was documented among patients with the same mutation, even within one kinship.4
Besides numerous recessive GDAP1 mutations (http://www.molgen.ua.ac.be/.CMTMutations/), 6 amino acid substitutions were shown to be pathogenic in a heterozygous state, indicating that GDAP1 mutations can be transmitted also as an autosomal dominant trait. Families were described with Ser34Cys, Arg120Trp, Gln218Glu, Arg226Ser, and Cys240Tyr mutations, while Thr157Pro occurred de novo in a single patient.9-12 The phenotype of the autosomal dominant GDAP1 patients described so far is consistent with mild axonal neuropathy, with later disease onset and slow progression, unlike most patients with recessive GDAP1 mutations.
Here we provide genetic and functional evidence for the pathogenicity of 4 heterozygous GDAP1 mutations and present detailed clinical description of the patients. Our study substantially broadens the understanding of autosomal dominant GDAP1-associated neuropathies.
METHODS
Patient cohort
We recruited 7 CMT2 families with dominant GDAP1 mutations via a call for autosomal dominant GDAP1 families within the International CMT Consortium. To ascertain dominant GDAP1 mutation frequency we additionally assembled a cohort of 97 index patients, belonging to unrelated families with dominantly inherited CMT. Families were selected if there were affected members in at least 2 generations. Our cohort included 38 patients diagnosed with CMT2, 17 with CMT1, and 9 with intermediate CMT. No distinct electrophysiologic categorization could be made for 33 patients. Routine mutation screening of the common dominant CMT genes was uneventful in the majority of patients.
Standard protocol approvals, registrations, and patient consents
All of our patients or their legal representatives signed an informed consent form prior to enrollment. This study was approved by the local institutional review board.
Mutation analysis
Total genomic DNA isolated from peripheral blood samples of patients with CMT and control individuals served as a template in the PCR reactions. All coding exons and exon-intron boundaries of GDAP1 were amplified using primer oligonucleotides described previously3 or redesigned with Primer313 (primer sequences and PCR conditions are available upon request). Subsequently, PCR products were purified with the Exonuclease I-Shrimp Alkaline Phosphatase enzymes (USB, Cleveland, OH) and bidirectionally sequenced using the BigDye® Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems, Foster City, CA). Electrophoretic separation of fragments was performed on an ABI3730xl DNA Analyzer (Applied Biosystems). Mutation analysis was conducted with the SeqMan™II (DNASTAR Inc., Madison, WI) program. Mutations were described according to the HGVS nomenclature (http://www.hgvs.org/mutnomen) with nucleotide numbering based on the published online protein (NP_061845) and mRNA (NM_018972) sequence of GDAP1 (www.ncbi.nlm.nih.gov). All sequence variants were confirmed by an independent PCR and resequencing of the original or newly obtained DNA samples. Segregation analysis of the mutations with the disease phenotype was performed in all available family members. For the newly identified His123Arg, Ala156Gly, and Pro274Leu mutations, 280 control individuals of European descent were screened. Additionally, 96 control individuals of Finnish origin were sequenced for the His123Arg mutation. The in silico prediction of the functional effect of mutations was performed with PolyPhen-2 algorithm (http://genetics.bwh.harvard.edu/pph2/index.shtml). Score 1 is the highest score in PolyPhen-2.
Multiplex amplicon quantification assay
We investigated the presence of intragenic deletions on the second allele of GDAP1 by the Multiplex Amplicon Quantification (MAQ) assay (www.multiplicon.com). A multiplex PCR was performed including 10 fluorescently labeled amplicons targeting the genomic region of GDAP1 and 6 reference amplicons located at randomly selected genomic positions outside the GDAP1 region and other known copy number variations. PCR fragments were mixed with a formamide and GeneScanTM 500 Liz® Size Standard (Applied Biosystems) solution (ratio 1:30) and size-separated on ABI3730xl DNA Analyzer. The ratio of peak areas between target and reference amplicons was calculated. Comparison of the normalized peak area values between patients and control individuals allowed determination of a dosage quotient (DQ) for each target amplicon, calculated by the MAQ-S package (www.multiplicon.com). DQ values below 0.75 were considered indicative for an amplicon deletion.
Haplotype and paternity testing
We ascertained haplotype sharing between families with common GDAP1 mutations with 6 highly informative short tandem repeat (STR) markers surrounding GDAP1 (D8S279, D8S286, D8S551, D8S1144, D8S548, D8S1829) and one exonic SNP (rs11554166). Paternity was examined with 15 STRs distributed throughout the genome (ATA38A05, D1S1646, D1S1653, D1S1360, D2S2256, D3S3037, D4S2382, D4S3240, D7S509, D8S1759, D9S1118, D12S1056, D12S2082, D16S2619, and GATA152H04). STRs were first PCR-amplified with fluorescently labeled primer pairs (sequences are available at www.ncbi.nlm.nih.gov); fragments were subsequently combined with a formamide and GeneScanTM 500 Liz® Size Standard (Applied Biosystems) mixture (ratio 1:30) and size-separated on an ABI3730xl DNA Analyzer. Genotyping results were analyzed with Local Genotype Viewer, an in-house developed software program (http://www.vibgeneticservicefacility.be/).
Functional assessment of pathogenicity of the Ala156Gly mutation
We transfected COS-7 or HeLa cells with cDNA constructs containing wild-type or mutant human GDAP1 forms as outlined previously.14 For transfection we used Fugene 6 reagent, according to the manufacturer’s recommendations (Roche). The detailed experimental procedures for evaluating the mitochondrial morphology of the transfected cells, the polyethylene glycol (PEG) cell fusion assay, and the cytochrome c release assay are as described previously.14,15
RESULTS
Genetic findings
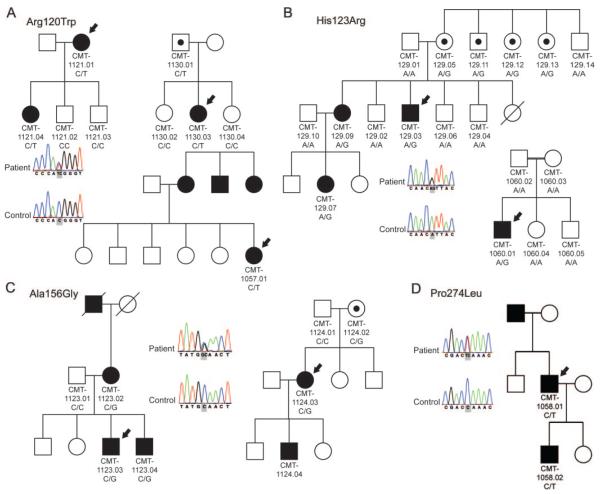
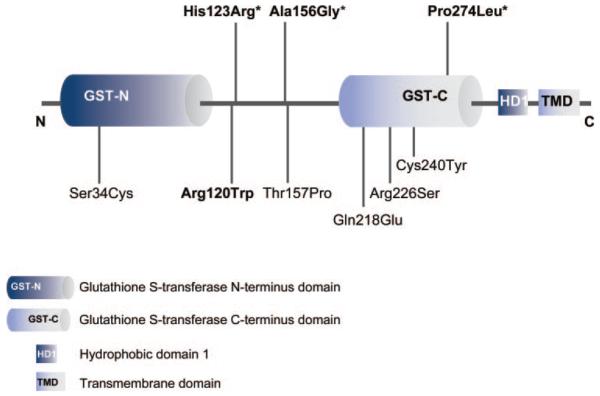
In this study, we identified 4 different heterozygous missense GDAP1 mutations in 8 unrelated CMT families (figure 1). We collected 7 of these families through collaborative efforts. One additional Finnish family (CMT-129) was identified through a GDAP1 mutation analysis of a heterogeneous cohort of 97 dominant CMT families and carried a new heterozygous c.358A>G (His123Arg) mutation. The same sequence variant occurred de novo in a patient of Tunisian origin (CMT-1060.01). Affected members of 2 Polish families (CMT-1123 and CMT-1124) carried a new heterozygous c.467C>G transversion in GDAP1 resulting in alanine to glycine change at codon 156 (Ala156Gly). Another novel missense variant c.821C>T (Pro274Leu) segregated with the CMT phenotype in an Italian family (CMT-1058). We identified a previously reported c.358C>T (Arg120Trp) mutation in families from Italy (CMT-1057), the United States (CMT-1130), and Austria (CMT-1121).10 All mutations target conserved amino acids and in silico analysis with PolypPhen-2 algorithm predicted a “probably damaging” effect on the protein function with score values ranging from 0.945 for Pro274Leu to 0.996 for His123Arg. The Pro274Leu mutation is localized in the C-terminal domain of GDAP1 protein, homologous to glutathione S-transferase domains (GST-N and GST-C) (figure 2). The other 3 mutations target the interdomain between GST-N and GST-C, which is in comparison to other GST families enlarged and therefore structurally characteristic for members of the GDAP1-family of GST proteins.16 The 3 novel mutations (His123Arg, Ala156Gly, Pro274Leu) were absent from 280 control individuals. We ruled out partial intragenic deletions on the second GDAP1 allele and thus a possible recessive inheritance by performing a MAQ assay.
Figure 1. Pedigrees of families with GDAP1 mutations.
Segregation analysis and sequence trace files are shown for 7 families. (A) Families CMT-1057, CMT-1121, and CMT-1130 carrying the Arg120Trp mutation. (B) Families CMT-129 and CMT-1060 with the His123Arg mutation. (C) Families CMT-1123 and CMT-1124 with the Ala156Gly mutation. (D) Family CMT-1058 with the Pro274Leu mutation. Square = male, circle = female, black filled symbol = affected, empty symbol = unaffected, empty symbol with black dot = unaffected mutation carrier, black arrow = proband; genotype is indicated under each individual from whom the DNA was available for testing.
Figure 2. Location of mutations in the GDAP1 protein.
The relative position of the mutations to the glutathione S-transferase (GST) homology region and interdomain region between GST-N and GST-C domains is indicated; bold = mutations identified in this study, * = novel mutations.
We traced segregation of the GDAP1 mutations in all available family members and all clinically affected individuals carried a mutant allele. This analysis further identified 4 unaffected carriers of the His123Arg mutation in the first generation of the Finnish family (CMT-129). The same His123Arg mutation however arose de novo and on a different chromosomal background in the isolated Tunisian patient CMT-1060.01 (table 1). Nonpenetrance was also documented for individual CMT-1124.02 carrying the Ala156Gly mutation. This mutation resided on the same haplotype in the affected members of the 2 Polish families (CMT-1123, CMT-1124), thus indicating a common ancestor (table 1). Additionally, we found one clinically normal but electrophysiologically affected carrier of the Pro274Leu mutation in family CMT-1058.
Table 1. Haplotype analysis of 8q21 markers in patients with autosomal dominant GDAP1 mutationsa.
| Arg120Trp |
His123Arg |
Ala156Gly |
|||||
|---|---|---|---|---|---|---|---|
| Marker | CMT-1121.01 | CMT-1130.03 | CMT-1057.01 | CMT-129.03 | CMT1060.01 | CMT-1123.03 | CMT-1124.03 |
| D8S279 | 251 | 229 | 229/245 | 241 | 238 | 235 | 229 |
| D8S286 | 232 | 230 | 232 | 232 | 242 | 230 | 230 |
| D8S551 | 263 | 253 | 263 | 253 | 253 | 275 | 275 |
| rs11554166 | G | G | G | G | T | G | G |
| D8S1144 | 161 | 161 | 157/165 | 169 | 157 | 157 | 157 |
| D8S548 | 230 | 230 | 230 | 230 | 242 | 234 | 234 |
| D8S1829 | 111 | 111 | 111 | 107 | 111 | 115 | 115 |
The alleles of the short tandem repeats are sized in base pairs (bp). The position of the markers is according to the reference assembly of NCBI genome build 36.3.
The 3 families (CMT-1057, CMT-1121, CMT-1130) carrying the Arg120Trp mutation share a disease haplotype and therefore represent one mutational event (table 1).
Pathogenicity of the Ala156Gly mutation
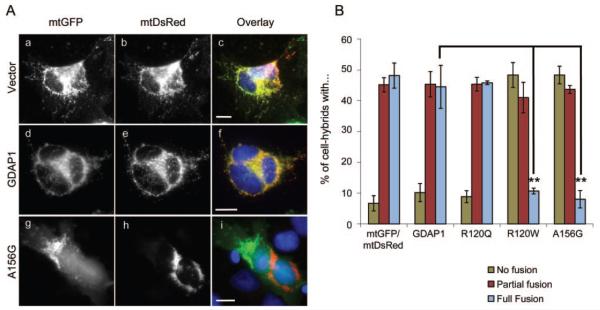
The Ala156Gly variation was found in 2 related Polish families and in one of them we identified an unaffected mutation carrier. We performed a cell fusion assay15 in order to rule out the possibility of Ala156Gly being a private, benign sequence variant cosegregating with the phenotype. Wild-type human GDAP1-transfected hybrids and hybrids expressing the recessively inherited mutation behaved in similar fashion as control transfected cells with no obvious inhibition of mitochondrial fusion (figure 3). These data were consistent with previous observations with the mouse GDAP1 and mutated forms hereof.15 Conversely, the fusion process was dramatically impaired in cells expressing the Ala156Gly variant or the known dominant Arg120Trp GDAP1 mutation (figure 3). Furthermore, impaired fusion process resulted in fragmented mitochondria in cells expressing the Ala156Gly mutant (figure e-1A on the Neurology® Web site at www.neurology.org) and increased sensitivity to proapoptotic agents, as quantified by a cytochrome c release assay, which was also in line with our previous findings for other dominant GDAP1 mutations (figure e-1, B and C).15
Figure 3. Dominant Ala156Gly mutation in GDAP1 impairs fusion of mitochondria.
(A) HeLa cells were transiently cotransfected with expression constructs encoding either mtDsRed or mtGFP (a-c), and wild-type human protein (d-f), autosomal recessively inherited (Arg120Gln), or dominantly inherited (Arg120Trp, Ala156Gly) (g-i) alleles of GDAP1. The cells were coplated and fused with PEG. Cell hybrids were stained for GDAP1 and analyzed for fusion of the mitochondrial markers mtGFP and mtDsRed. Bars, 10 μm. (B) Quantitation analysis revealed that fusion was similar for controls, GDAP1-transfected hybrids, and hybrids expressing the recessively inherited Arg120Gln. A dramatically impaired fusion was observed for cell hybrids expressing Arg120Trp and Ala156Gly (n = 3, average and standard error are shown, statistically significant levels are shown for the category full fusion; Student t test p value ** <0.005).
Clinical findings
Detailed clinical and electrophysiologic studies were performed in a total of 15 patients from 8 families with dominant GDAP1 mutations. Six additional patients were affected by history; however, either DNA samples or clinical details were not available for this study (see table 2, figure 1, table e-1, and table e-2).
Table 2. Overview of clinical findings in families with dominant GDAP1 mutations identified in this study.
| Mutation and family |
Ethnicity | Diagnosis | AAO | No. of affected |
No. of asymptomatic mutation carriers |
Remarks |
|---|---|---|---|---|---|---|
| Arg120Trp | ||||||
| CMT-1057 | Italian | CMT2 | 20 y | 4 | – | – |
| CMT-1121 | Austrian | CMT2 | 10-45 y | 2 | – | Proximal weakness in UL/LL in patient CMT-1121.01 |
| CMT-1130 | American (Ashkenazi Jewish) |
CMT2 | Childhood | 1 | 1 | Proximal weakness LL in patient CMT-1130.03 |
| His123Arg | ||||||
| CMT-129 | Finnish | CMT2 | 3 to > 32 y | 3 | 4 | Proximal weakness LL in patient CMT-129.07 |
| CMT-1060 | Tunisian | CMT-INT | Childhood | 1 | – | Delayed early motor milestones (independent gait at 24 mo), severely affected |
| Ala156Gly | ||||||
| CMT-1123 | Polish | CMT2 | 8-13 y | 4 | – | Cardiac arrhythmia cosegregating in family |
| CMT-1124 | Polish | CMT2 | 13-18 y | 2 | 1 | – |
| Pro274Leu | ||||||
| CMT-1058 | Italian | CMT-INT, CMT2 |
47 y | 3 | – | Fast disease progression, wheelchair-bound at 61 y (CMT-1058.01); CMT-1058.02 clinically normal, electrophysiology: clear axonal neuropathy |
Abbreviations: AAO = age at onset; INT = intermediate; LL = lower limb; UL = upper limb.
We observed considerable phenotypic variability among these individuals even though many carried the same mutation. Based on electrophysiologic data, the majority of our autosomal dominant GDAP1 patients can be diagnosed as axonal CMT; patients CMT-1060.01 and CMT-1058.01 had nerve conduction velocities (NCVs) in the intermediate range. Examination of a sural nerve biopsy in the same patient showed mixed axonal and demyelinating features.
Disease onset varied widely, ranging from childhood to late adulthood. Walking difficulties were the most common initial symptoms. Disease progression was slow and as a rule patients remained ambulatory. Four patients (CMT-129.03, CMT-129.07, CMT-1060.01, and CMT-1130.03) required an ankle foot orthosis; patient CMT-1058.01 became wheelchair-dependent at the age of 61 years. Weakness and atrophy was mainly restricted to distal muscles of the lower and upper limbs except for patients CMT-129.03, CMT-129.07, CMT-1058.01, and CMT-1130.03, who had mild signs of proximal weakness.
We did not observe vocal fold involvement in this study; patient CMT-1124.03 presented with hoarse-ness but a laryngoscopic evaluation could not be performed. We noted no additional clinical features in our patients apart from thoracic scoliosis seen in 2 sibs, CMT-1123.03 and CMT-1123.04. Noteworthy are several mildly affected or even asymptomatic mutation carriers at an advanced age (CMT-129.05, CMT-129.11, CMT-129.12, CMT-129.13, CMT-1058.02, CMT-1124.02, and CMT-1130.01).
DISCUSSION
Mutations in GDAP1 are most commonly associated with ARCMT; however, rare disease-causing heterozygous missense mutations have been reported in recent years.9-12 GDAP1 is thus one of the few CMT-associated genes for which both autosomal dominant and recessive inheritance patterns can be observed. Other notable examples are early growth response 2 (EGR2) and neurofilament light polypeptide (NEFL).17-23 Interestingly, the mode of mutation inheritance was demonstrated to be dependent on the disease mechanism for all 3 genes.15,18,23,24
Here, we present genetic and functional evidence and comprehensive clinical data in a large collection of patients with CMT with dominant GDAP1 mutations. In addition to the dominant GDAP1 families gathered through international collaboration, we screened a group of 97 dominant CMT families and identified one new heterozygous mutation. This indicates that the frequency of dominant GDAP1 mutations in larger cohorts is relatively low, at about 1%. Conversely, mutations in GDAP1 are common in cohorts of recessive CMT families.25 The recently reported mutation frequency of 27% in a small Italian ADCMT cohort is considerably higher than in this study, possibly because our study population is larger and more heterogeneous both clinically and ethnically.9
We identified 4 autosomal dominant GDAP1 mutations in 8 families. Disease-haplotype sharing suggested that the previously described Arg120Trp mutation is a founder mutation. In a Finnish pedigree we identified a novel His123Arg mutation that also arose as a de novo event in an isolated Tunisian patient. Haplotype analysis showed that this mutation occurred independently on different genetic backgrounds thus underscoring its pathogenicity (table 1). Conversely, a novel heterozygous Ala156Gly variation (figure 1) was found in 2 Polish families that were distantly related (table 1). In addition, pathogenicity was uncertain due to an asymptomatic female mutation carrier in one of these families (CMT-1124) and the cosegregation of a non-CMT specific clinical feature (cardiac arrhythmia) in the second (CMT-1123). Therefore we performed functional characterization of the Ala156Gly mutation.15
GDAP1 has sequence similarity to GSTs but neither binding to glutathione nor GST activity could be demonstrated.26,27 Notably, GDAP1 is an integral mitochondrial outer membrane protein playing an important role in mitochondrial dynamics and functioning.14,26 Overexpression of GDAP1 induces mitochondrial fission, a process abrogated to some extent by recessive GDAP1 missense mutations, resulting in a mixture of fragmented and enlarged tubular mitochondria.14 Conversely, dominant mutations in GDAP1 alter the mitochondrial fusion process, as we have recently shown.15 These clear differences in mitochondrial dynamics between dominant and recessive mutations prompted us to test the effect of the novel Ala156Gly variant. We demonstrated that this variant impairs the mitochondrial fusion process (figure 3) leading to fragmented mitochondria (figure e-1A) and increased cell sensitivity to apoptosis (figure e-1, B and C) in a similar fashion as other known dominant GDAP1 mutations, thus supporting the pathogenic nature of this variant.
The mutations identified in our study target highly conserved residues that are predicted to be located in α-helices.16 The Arg120Trp mutation was previously identified in other studies, making it the most frequently observed dominant GDAP1 mutation so far. Surprisingly, in our study all families with this variant shared a common ancestor. Conversely, His123Arg was proven to be recurrent, as it occurs on different chromosomal backgrounds. The recurrent character of some dominant GDAP1 mutations suggests presence of mutational hotspots in the GDAP1 gene. Interestingly, all known dominant GDAP1 mutations target residues within the GST-homology region of the GDAP1-protein family. Recessive GDAP1 mutations in contrast are spread throughout the protein sequence. These data under-score the functional importance of the GST-like domain and may imply that the location of the mutation has an impact on the molecular mechanism leading to the ADCMT phenotype.
The dominant GDAP1 mutations described so far are usually associated with mild axonal neuropathy with late onset in the second or beginning of the third decade of life and slow disease evolution.10-12 The age at onset in half of our patient group was before the end of the first decade of life, which is earlier than noted previously. Disease progression in the majority of the patients in the current study is slow and most of them remain ambulatory to date. Although we observed proximal weakness in some patients, generally its extent and severity is less pronounced than for typical recessive GDAP1 mutations. A notable exception is patient CMT-1058.01 who developed symptoms only at the age of 47 years, but nonetheless displayed a rapid deterioration resulting in wheelchair dependency at the age of 61 years. An acquired neuropathy was suspected; however, CSF studies remained negative and no clinical improvement was seen after various immunomodulating treatments.
GDAP1 is one of the few CMT genes28-33 that can be associated with VFP,8,34 a severity hallmark for autosomal recessive GDAP1 neuropathies.8 One mildly affected individual (CMT-1124.03) from our cohort presented with a hoarse voice but VFP was not formally diagnosed. The general absence of VFP in autosomal dominant GDAP1 is another indication of the generally milder phenotype.
In our study, electrophysiologic findings are quite heterogeneous. We observed a clear axonal neuropathy (i.e., moderately slowed NCVs >38 m/s and decreased CMAP amplitudes) in several patients but not in others. In addition, clinical variability is very striking, even within one family. In that respect pedigree CMT-129 (His123Arg mutation) is exemplary; the phenotype varied from asymptomatic individuals (CMT-129.05, CMT-129.11, CMT-129.12, and CMT-129.13) to patient CMT129.03, who displayed a clinically and electrophysiologically more pronounced phenotype. Overall, however, individuals from this family had the least abnormal NCVs. Reduced penetrance, though not uncommon for dominant GDAP1 mutations, is generally rare in the context of CMT and is only occasionally observed for mutations in MPZ, GARS, BSCL2, and TRPV4.33 Pronounced phenotypic variability is thus an important common characteristic of dominant GDAP1 mutations. Clinicians evaluating patients in terms of consideration of this diagnosis should therefore take into account that some mutation carriers only have mild or minimal clinical and electrophysiologic abnormalities. A diagnosis should be based on detailed electrophysiologic findings in combination with the clinical and genetic data in the proband and extended to the proband’s family.
In this study, we present a large cohort of patients with dominant GDAP1 mutations, broadening the current knowledge about the phenotypic and genetic spectrum of this type of neuropathy. Based on our findings, it is advisable to screen for mutations in GDAP1 in ARCMT patients, but also ADCMT families or isolated mildly affected patients with variable electrophysiology in the absence of additional clinical features like VFP or scoliosis. Sequencing GDAP1 may yield heterozygous sequence variants without clear autosomal dominant segregation due to the common occurrence of reduced penetrance. In fact, several additional missense variations of unknown significance were encountered when performing diagnostic GDAP1 screening in isolated patients or small families (P. De Jonghe, personal communication, 2010). Identified sequence variants can be regarded as disease-causing when a clear segregation with the phenotype is present or other strong genetic evidence of pathogenicity such as recurrent character, absence from controls, and targeting conserved and functionally relevant residues. If the genetic data remain unconvincing, a functional assay may be considered in order to distinguish truly pathogenic sequence variants from benign polymorphisms, like for the Ala156Gly mutation.
ACKNOWLEDGMENT
The authors thank the patients and their families for their willingness to participate in this study.
Study funding: This study was supported by the University of Antwerp, the Fund for Scientific Research (FWO-Flanders, grant G017209N), the “Association Belge contre les Maladies Neuromusculaires” (ABMM), the Medical Foundation Queen Elisabeth (GSKE), the Interuniversity Attraction Poles P6/43 program of the Belgian Federal Science Policy Office (BELSPO), the “Methusalem excellence grant” of the Flemish Government, and the Austrian Science Fond (FWF, P19455-B05). M.Z. and J.B. are supported by PhD fellowships of the FWO-Flanders. This study was also supported by the Polish Ministry of Science and Higher Education (grant No. NN 402 27 63 36) to A.K. Work in the laboratory of U.S. is supported by the Swiss National Science Foundation and the NCCR Neural Plasticity and Repair.
GLOSSARY
- ADCMT
autosomal dominant Charcot-Marie-Tooth
- ARCMT
autosomal recessive Charcot-Marie-Tooth
- CMT
Charcot-Marie-Tooth
- DQ
dosage quotient
- GDAP1
ganglioside-induced differentiation-associated protein 1
- MAQ
Multiplex Amplicon Quantification
- NCV
nerve conduction velocity
- PEG
polyethylene glycol
- STR
short tandem repeat
- VFP
vocal fold paresis
Footnotes
DISCLOSURE
M. Zimoń, Dr. Baets, Dr. Fabrizi, Dr. Jaakkola, Dr. Kabzinska, Dr. Pilch, and A.B. Schindler report no disclosures. Dr. Cornblath serves/has served on scientific advisory boards for Ardea Biosciences, Inc., Avigen, Inc., Pfizer Inc, Johnson & Johnson, GlaxoSmithKline, Abbott, Acorda Therapeutics Inc., Alexion Pharmaceuticals, Inc., Astellas Pharma Inc., Baxter International Inc., Bionevia Pharmaceuticals Inc., Bristol-Myers Squibb, Cebix, CSL Behring, Eisai Inc., Exelixis Inc., FoldRx, Genzyme Corporation, Keryx Biopharmaceuticals, Inc., Mitsubishi Tanabe Pharma Corporation, Octapharma AG, Sangamo BioSciences, Inc., sanofi-aventis, and Talecris Biotherapeutics; holds and has received license fee payments for patents re: Total neuropathy score (nurse, clinical), Methods to assess neuropathy. Dr. Fischbeck receives intramural research support from the NIH/NINDS. Dr. Auer-Grumbach, Dr. Guelly, N. Huber, E. De Vriendt, and Dr. Timmerman report no disclosures. Dr. Suter receives research support from the Swiss National Science Foundation and the NCCR Neural Plasticity and Repair. Dr. Hausmanowa-Petrusewicz, Dr. Niemann, Dr. Kochanski, Dr. De Jonghe, and Dr. Jordanova report no disclosures.
REFERENCES
- 1.Skre H. Genetic and clinical aspects of Charcot-Marie-Tooth’s disease. Clin Genet. 1974;6:98–118. doi: 10.1111/j.1399-0004.1974.tb00638.x. [DOI] [PubMed] [Google Scholar]
- 2.Baxter RV, Ben OK, Rochelle JM, et al. Ganglioside-induced differentiation-associated protein-1 is mutant in Charcot-Marie-Tooth disease type 4A/8q21. Nat Genet. 2002;30:21–22. doi: 10.1038/ng796. [DOI] [PubMed] [Google Scholar]
- 3.Cuesta A, Pedrola L, Sevilla T, et al. The gene encoding ganglioside-induced differentiation-associated protein 1 is mutated in axonal Charcot-Marie-Tooth type 4A disease. Nat Genet. 2002;30:22–25. doi: 10.1038/ng798. [DOI] [PubMed] [Google Scholar]
- 4.Nelis E, Erdem S, Van Den Bergh PY, et al. Mutations in GDAP1: autosomal recessive CMT with demyelination and axonopathy. Neurology. 2002;59:1865–1872. doi: 10.1212/01.wnl.0000036272.36047.54. [DOI] [PubMed] [Google Scholar]
- 5.Senderek J, Bergmann C, Ramaekers VT, et al. Mutations in the ganglioside-induced differentiation-associated protein-1 (GDAP1) gene in intermediate type autosomal recessive Charcot-Marie-Tooth neuropathy. Brain. 2003;126:642–649. doi: 10.1093/brain/awg068. [DOI] [PubMed] [Google Scholar]
- 6.Bernard R, De Sandre-Giovannoli A, Delague V, Levy N. Molecular genetics of autosomal-recessive axonal Charcot-Marie-Tooth neuropathies. Neuromolecular Med. 2006;8:87–106. doi: 10.1385/nmm:8:1-2:87. [DOI] [PubMed] [Google Scholar]
- 7.Dubourg O, Azzedine H, Verny C, et al. Autosomal-recessive forms of demyelinating Charcot-Marie-Tooth disease. Neuromolecular Med. 2006;8:75–86. doi: 10.1385/nmm:8:1-2:75. [DOI] [PubMed] [Google Scholar]
- 8.Sevilla T, Jaijo T, Nauffal D, et al. Vocal cord paresis and diaphragmatic dysfunction are severe and frequent symptoms of GDAP1-associated neuropathy. Brain. 2008;131:3051–3061. doi: 10.1093/brain/awn228. [DOI] [PubMed] [Google Scholar]
- 9.Crimella C, Tonelli A, Airoldi G, et al. The GST domain of GDAP1 is a frequent target of mutations in the dominant form of axonal Charcot Marie Tooth type 2K. J Med Genet. 2010;47:712–716. doi: 10.1136/jmg.2010.077909. [DOI] [PubMed] [Google Scholar]
- 10.Claramunt R, Pedrola L, Sevilla T, et al. Genetics of Charcot-Marie-Tooth disease type 4A: mutations, inheritance, phenotypic variability, and founder effect. J Med Genet. 2005;42:358–365. doi: 10.1136/jmg.2004.022178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chung KW, Kim SM, Sunwoo IN, et al. A novel GDAP1 Q218E mutation in autosomal dominant Charcot-Marie-Tooth disease. J Hum Genet. 2008;53:360–364. doi: 10.1007/s10038-008-0249-3. [DOI] [PubMed] [Google Scholar]
- 12.Cassereau J, Chevrollier A, Gueguen N, et al. Mitochondrial complex I deficiency in GDAP1-related autosomal dominant Charcot-Marie-Tooth disease (CMT2K) Neurogenetics. 2009;10:145–150. doi: 10.1007/s10048-008-0166-9. [DOI] [PubMed] [Google Scholar]
- 13.Rozen S, Skaletsky H. Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol. 2000;132:365–386. doi: 10.1385/1-59259-192-2:365. [DOI] [PubMed] [Google Scholar]
- 14.Niemann A, Ruegg M, La Padula V, Schenone A, Suter U. Ganglioside-induced differentiation associated protein 1 is a regulator of the mitochondrial network: new implications for Charcot-Marie-Tooth disease. J Cell Biol. 2005;170:1067–1078. doi: 10.1083/jcb.200507087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Niemann A, Wagner KM, Ruegg M, Suter U. GDAP1 mutations differ in their effects on mitochondrial dynamics and apoptosis depending on the mode of inheritance. Neurobiol Dis. 2009;36:509–520. doi: 10.1016/j.nbd.2009.09.011. [DOI] [PubMed] [Google Scholar]
- 16.Marco A, Cuesta A, Pedrola L, Palau F, Marin I. Evolutionary and structural analyses of GDAP1, involved in Charcot-Marie-Tooth disease, characterize a novel class of glutathione transferase-related genes. Mol Biol Evol. 2004;21:176–187. doi: 10.1093/molbev/msh013. [DOI] [PubMed] [Google Scholar]
- 17.Warner LE, Mancias P, Butler IJ, et al. Mutations in the early growth response 2 (EGR2) gene are associated with hereditary myelinopathies. Nat Genet. 1998;18:382–384. doi: 10.1038/ng0498-382. [DOI] [PubMed] [Google Scholar]
- 18.Warner LE, Svaren J, Milbrandt J, Lupski JR. Functional consequences of mutations in the early growth response 2 gene (EGR2) correlate with severity of human myelinopathies. Hum Mol Genet. 1999;8:1245–1251. doi: 10.1093/hmg/8.7.1245. [DOI] [PubMed] [Google Scholar]
- 19.Mersiyanova IV, Perepelov AV, Polyakov AV, et al. A new variant of Charcot-Marie-Tooth disease type 2 is probably the result of a mutation in the neurofilament-light gene. Am J Hum Genet. 2000;67:37–46. doi: 10.1086/302962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De JP, Mersivanova I, Nelis E, et al. Further evidence that neurofilament light chain gene mutations can cause Charcot-Marie-Tooth disease type 2E. Ann Neurol. 2001;49:245–249. doi: 10.1002/1531-8249(20010201)49:2<245::aid-ana45>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 21.Jordanova A, De JP, Boerkoel CF, et al. Mutations in the neurofilament light chain gene (NEFL) cause early onset severe Charcot-Marie-Tooth disease. Brain. 2003;126:590–597. doi: 10.1093/brain/awg059. [DOI] [PubMed] [Google Scholar]
- 22.Abe A, Numakura C, Saito K, et al. Neurofilament light chain polypeptide gene mutations in Charcot-Marie-Tooth disease: nonsense mutation probably causes a recessive phenotype. J Hum Genet. 2009;54:94–97. doi: 10.1038/jhg.2008.13. [DOI] [PubMed] [Google Scholar]
- 23.Yum SW, Zhang J, Mo K, Li J, Scherer SS. A novel recessive Nefl mutation causes a severe, early-onset axonal neuropathy. Ann Neurol. 2009;66:759–770. doi: 10.1002/ana.21728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perez-Olle R, Lopez-Toledano MA, Goryunov D, et al. Mutations in the neurofilament light gene linked to Charcot-Marie-Tooth disease cause defects in transport. J Neurochem. 2005;93:861–874. doi: 10.1111/j.1471-4159.2005.03095.x. [DOI] [PubMed] [Google Scholar]
- 25.Ammar N, Nelis E, Merlini L, et al. Identification of novel GDAP1 mutations causing autosomal recessive Charcot-Marie-Tooth disease. Neuromuscul Disord. 2003;13:720–728. doi: 10.1016/s0960-8966(03)00093-2. [DOI] [PubMed] [Google Scholar]
- 26.Pedrola L, Espert A, Wu X, Claramunt R, Shy ME, Palau F. GDAP1, the protein causing Charcot-Marie-Tooth disease type 4A, is expressed in neurons and is associated with mitochondria. Hum Mol Genet. 2005;14:1087–1094. doi: 10.1093/hmg/ddi121. [DOI] [PubMed] [Google Scholar]
- 27.Shield AJ, Murray TP, Board PG. Functional characterisation of ganglioside-induced differentiation-associated protein 1 as a glutathione transferase. Biochem Biophys Res Commun. 2006;347:859–866. doi: 10.1016/j.bbrc.2006.06.189. [DOI] [PubMed] [Google Scholar]
- 28.Thomas PK, Marques W, Jr, Davis MB, et al. The phenotypic manifestations of chromosome 17p11.2 duplication. Brain. 1997;120:465–478. doi: 10.1093/brain/120.3.465. [DOI] [PubMed] [Google Scholar]
- 29.Pareyson D, Taroni F, Botti S, et al. Cranial nerve involvement in CMT disease type 1 due to early growth response 2 gene mutation. Neurology. 2000;54:1696–1698. doi: 10.1212/wnl.54.8.1696. [DOI] [PubMed] [Google Scholar]
- 30.Puls I, Jonnakuty C, LaMonte BH, et al. Mutant dynactin in motor neuron disease. Nat Genet. 2003;33:455–456. doi: 10.1038/ng1123. [DOI] [PubMed] [Google Scholar]
- 31.Puls I, Oh SJ, Sumner CJ, et al. Distal spinal and bulbar muscular atrophy caused by dynactin mutation. Ann Neurol. 2005;57:687–694. doi: 10.1002/ana.20468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McEntagart M, Norton N, Williams H, et al. Localization of the gene for distal hereditary motor neuronopathy VII (dHMN-VII) to chromosome 2q14. Am J Hum Genet. 2001;68:1270–1276. doi: 10.1086/320122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zimon M, Baets J, Auer-Grumbach M, et al. Dominant mutations in the cation channel gene transient receptor potential vanilloid 4 cause an unusual spectrum of neuropathies. Brain. 2010;133:1798–1809. doi: 10.1093/brain/awq109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stojkovic T, Latour P, Viet G, et al. Vocal cord and diaphragm paralysis, as clinical features of a French family with autosomal recessive Charcot-Marie-Tooth disease, associated with a new mutation in the GDAP1 gene. Neuromuscul Disord. 2004;14:261–264. doi: 10.1016/j.nmd.2004.01.003. [DOI] [PubMed] [Google Scholar]